Neuroactive Steroids, Toll-like Receptors, and Neuroimmune Regulation: Insights into Their Impact on Neuropsychiatric Disorders
- PMID: 38792602
- PMCID: PMC11122352
- DOI: 10.3390/life14050582
Neuroactive Steroids, Toll-like Receptors, and Neuroimmune Regulation: Insights into Their Impact on Neuropsychiatric Disorders
Abstract
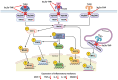
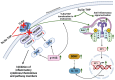
Pregnane neuroactive steroids, notably allopregnanolone and pregnenolone, exhibit efficacy in mitigating inflammatory signals triggered by toll-like receptor (TLR) activation, thus attenuating the production of inflammatory factors. Clinical studies highlight their therapeutic potential, particularly in conditions like postpartum depression (PPD), where the FDA-approved compound brexanolone, an intravenous formulation of allopregnanolone, effectively suppresses TLR-mediated inflammatory pathways, predicting symptom improvement. Additionally, pregnane neurosteroids exhibit trophic and anti-inflammatory properties, stimulating the production of vital trophic proteins and anti-inflammatory factors. Androstane neuroactive steroids, including estrogens and androgens, along with dehydroepiandrosterone (DHEA), display diverse effects on TLR expression and activation. Notably, androstenediol (ADIOL), an androstane neurosteroid, emerges as a potent anti-inflammatory agent, promising for therapeutic interventions. The dysregulation of immune responses via TLR signaling alongside reduced levels of endogenous neurosteroids significantly contributes to symptom severity across various neuropsychiatric disorders. Neuroactive steroids, such as allopregnanolone, demonstrate efficacy in alleviating symptoms of various neuropsychiatric disorders and modulating neuroimmune responses, offering potential intervention avenues. This review emphasizes the significant therapeutic potential of neuroactive steroids in modulating TLR signaling pathways, particularly in addressing inflammatory processes associated with neuropsychiatric disorders. It advances our understanding of the complex interplay between neuroactive steroids and immune responses, paving the way for personalized treatment strategies tailored to individual needs and providing insights for future research aimed at unraveling the intricacies of neuropsychiatric disorders.
Keywords: allopregnanolone; cytokines; inflammation; neurosteroid; pregnenolone.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

