Opportunistic Pathogens in Drinking Water Distribution Systems-A Review
- PMID: 38792751
- PMCID: PMC11124194
- DOI: 10.3390/microorganisms12050916
Opportunistic Pathogens in Drinking Water Distribution Systems-A Review
Abstract
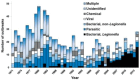
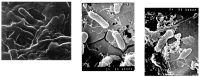
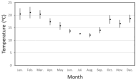
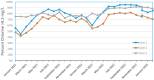
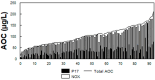
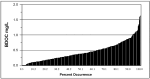
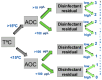
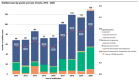
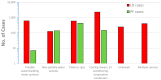
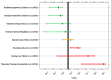
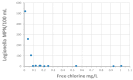
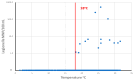
In contrast to "frank" pathogens, like Salmonella entrocolitica, Shigella dysenteriae, and Vibrio cholerae, that always have a probability of disease, "opportunistic" pathogens are organisms that cause an infectious disease in a host with a weakened immune system and rarely in a healthy host. Historically, drinking water treatment has focused on control of frank pathogens, particularly those from human or animal sources (like Giardia lamblia, Cryptosporidium parvum, or Hepatitis A virus), but in recent years outbreaks from drinking water have increasingly been due to opportunistic pathogens. Characteristics of opportunistic pathogens that make them problematic for water treatment include: (1) they are normally present in aquatic environments, (2) they grow in biofilms that protect the bacteria from disinfectants, and (3) under appropriate conditions in drinking water systems (e.g., warm water, stagnation, low disinfectant levels, etc.), these bacteria can amplify to levels that can pose a public health risk. The three most common opportunistic pathogens in drinking water systems are Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. This report focuses on these organisms to provide information on their public health risk, occurrence in drinking water systems, susceptibility to various disinfectants, and other operational practices (like flushing and cleaning of pipes and storage tanks). In addition, information is provided on a group of nine other opportunistic pathogens that are less commonly found in drinking water systems, including Aeromonas hydrophila, Klebsiella pneumoniae, Serratia marcescens, Burkholderia pseudomallei, Acinetobacter baumannii, Stenotrophomonas maltophilia, Arcobacter butzleri, and several free-living amoebae including Naegleria fowleri and species of Acanthamoeba. The public health risk for these microbes in drinking water is still unclear, but in most cases, efforts to manage Legionella, mycobacteria, and Pseudomonas risks will also be effective for these other opportunistic pathogens. The approach to managing opportunistic pathogens in drinking water supplies focuses on controlling the growth of these organisms. Many of these microbes are normal inhabitants in biofilms in water, so the attention is less on eliminating these organisms from entering the system and more on managing their occurrence and concentrations in the pipe network. With anticipated warming trends associated with climate change, the factors that drive the growth of opportunistic pathogens in drinking water systems will likely increase. It is important, therefore, to evaluate treatment barriers and management activities for control of opportunistic pathogen risks. Controls for primary treatment, particularly for turbidity management and disinfection, should be reviewed to ensure adequacy for opportunistic pathogen control. However, the major focus for the utility's opportunistic pathogen risk reduction plan is the management of biological activity and biofilms in the distribution system. Factors that influence the growth of microbes (primarily in biofilms) in the distribution system include, temperature, disinfectant type and concentration, nutrient levels (measured as AOC or BDOC), stagnation, flushing of pipes and cleaning of storage tank sediments, and corrosion control. Pressure management and distribution system integrity are also important to the microbial quality of water but are related more to the intrusion of contaminants into the distribution system rather than directly related to microbial growth. Summarizing the identified risk from drinking water, the availability and quality of disinfection data for treatment, and guidelines or standards for control showed that adequate information is best available for management of L. pneumophila. For L. pneumophila, the risk for this organism has been clearly established from drinking water, cases have increased worldwide, and it is one of the most identified causes of drinking water outbreaks. Water management best practices (e.g., maintenance of a disinfectant residual throughout the distribution system, flushing and cleaning of sediments in pipelines and storage tanks, among others) have been shown to be effective for control of L. pneumophila in water supplies. In addition, there are well documented management guidelines available for the control of the organism in drinking water distribution systems. By comparison, management of risks for Mycobacteria from water are less clear than for L. pneumophila. Treatment of M. avium is difficult due to its resistance to disinfection, the tendency to form clumps, and attachment to surfaces in biofilms. Additionally, there are no guidelines for management of M. avium in drinking water, and one risk assessment study suggested a low risk of infection. The role of tap water in the transmission of the other opportunistic pathogens is less clear and, in many cases, actions to manage L. pneumophila (e.g., maintenance of a disinfectant residual, flushing, cleaning of storage tanks, etc.) will also be beneficial in helping to manage these organisms as well.
Keywords: climate change; disinfection; distribution systems; drinking water; flushing; legionella pneumophila; mycobacterium; opportunistic pathogens; pseudomonas; water treatment.
Conflict of interest statement
Author Mark W. LeChevallier was employed by the company Dr. Water Consulting, LLC. Authors Toby Prosser and Melita Stevens were employed by the company Melbourne Water. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- National Health and Medical Research Council (NHMRC) Australian Drinking Water Guidelines 6. Version 3.8. 6 National Water Quality Management Strategy. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra. [(accessed on 24 April 2024)];2022 Available online: https://www.nhmrc.gov.au/about-us/publications/australian-drinking-water....
-
- Baker M.N. The Quest for Pure Water: The History of Water Purification from the Earliest Records to the Twentieth Century. American Water Works Assn; Denver, CO, USA: 1948.
-
- Gerba C.P., Rose J.B. Viruses in Source and Drinking Water. In: McFeters G.A., editor. Drinking Water Microbiology. Springer; New York, NY, USA: 1990. Brock/Springer Series in Contemporary Bioscience. - DOI
-
- LeChevallier M.W., Norton W.D. Occurrence of Giardia and Cryptosporidium in Raw and Finished Drinking Water. J. Amer. Water Works Assoc. 1995;87:54–68. doi: 10.1002/j.1551-8833.1995.tb06422.x. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

