Exploring the Toxin-Mediated Mechanisms in Clostridioides difficile Infection
- PMID: 38792835
- PMCID: PMC11124097
- DOI: 10.3390/microorganisms12051004
Exploring the Toxin-Mediated Mechanisms in Clostridioides difficile Infection
Abstract
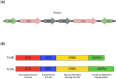
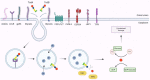
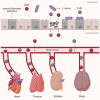
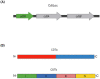
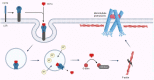
Clostridioides difficile infection (CDI) is the leading cause of nosocomial antibiotic-associated diarrhea, and colitis, with increasing incidence and healthcare costs. Its pathogenesis is primarily driven by toxins produced by the bacterium C. difficile, Toxin A (TcdA) and Toxin B (TcdB). Certain strains produce an additional toxin, the C. difficile transferase (CDT), which further enhances the virulence and pathogenicity of C. difficile. These toxins disrupt colonic epithelial barrier integrity, and induce inflammation and cellular damage, leading to CDI symptoms. Significant progress has been made in the past decade in elucidating the molecular mechanisms of TcdA, TcdB, and CDT, which provide insights into the management of CDI and the future development of novel treatment strategies based on anti-toxin therapies. While antibiotics are common treatments, high recurrence rates necessitate alternative therapies. Bezlotoxumab, targeting TcdB, is the only available anti-toxin, yet limitations persist, prompting ongoing research. This review highlights the current knowledge of the structure and mechanism of action of C. difficile toxins and their role in disease. By comprehensively describing the toxin-mediated mechanisms, this review provides insights for the future development of novel treatment strategies and the management of CDI.
Keywords: Clostridioides difficile; actin cytoskeleton; bacterial toxins; infection; inflammation; pathogenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Warny M., Pepin J., Fang A., Killgore G., Thompson A., Brazier J., Frost E., McDonald L.C. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/s0140-6736(05)67420-x. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

