Clinical Evaluation of Oral Midazolam Containing Cyclodextrin in Pediatric Magnetic Resonance: A Retrospective Cohort Study
- PMID: 38793054
- PMCID: PMC11122387
- DOI: 10.3390/jpm14050472
Clinical Evaluation of Oral Midazolam Containing Cyclodextrin in Pediatric Magnetic Resonance: A Retrospective Cohort Study
Abstract
Background: Reducing a child's level of anxiety before magnetic resonance imaging (MRI) procedures allows for better behavioral outcomes. The aim of this retrospective study was to evaluate anxiolytic efficacy of Midazolam/γ-cyclodextrin oral formulation.
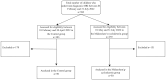
Methods: We retrospectively reviewed 100 medical charts of children who, between 1 February and 31 July 2022, underwent MRI under general anesthesia with or without premedication with midazolam/γ-cyclodextrin. Primary outcome was comparison of behavior to facemask positioning, while secondary endpoints were degree of drugs acceptance, anxiolytic effect evaluation, child's behavior on separation, and sevoflurane need.
Results: Facemask positioning was accepted by 58% of the midazolam/γ-cyclodextrin group compared to 22% of children in the control group. The rate of acceptance was >90%. At the moment of separation from parent, none of the premedicated children needed to be restrained compared to 18% in the control group. A lower percentage of sevoflurane was needed for eye-closure at induction of anesthesia and for anesthesia maintenance. At emergence from anesthesia, 46% of children in the premedicated group compared to 66% of children in the control group showed transient agitation.
Conclusions: Midazolam/γ-cyclodextrin showed a good profile of acceptance, satisfactory anxiolytic properties, and reduced need for anesthetics when administered to children before MRI under general anesthesia.
Keywords: behavior change; emergence agitation; emergence delirium; general anesthesia; magnetic resonance imaging; oral midazolam; premedication.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Comparison of oral dexmedetomidine and midazolam for premedication and emergence delirium in children after dental procedures under general anesthesia: a retrospective study.Drug Des Devel Ther. 2018 Mar 28;12:647-653. doi: 10.2147/DDDT.S163828. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 29636599 Free PMC article.
-
Effects of hydroxyzine-midazolam premedication on sevoflurane-induced paediatric emergence agitation: a prospective randomised clinical trial.Eur J Anaesthesiol. 2011 Sep;28(9):640-5. doi: 10.1097/EJA.0b013e328344db1a. Eur J Anaesthesiol. 2011. PMID: 21822077 Clinical Trial.
-
Premedication with low-dose oral midazolam reduces the incidence and severity of emergence agitation in pediatric patients following sevoflurane anesthesia.Acta Anaesthesiol Sin. 2001 Dec;39(4):169-77. Acta Anaesthesiol Sin. 2001. PMID: 11840583 Clinical Trial.
-
Oral Dexmedetomidine Achieves Superior Effects in Mitigating Emergence Agitation and Demonstrates Comparable Sedative Effects to Oral Midazolam for Pediatric Premedication: A Systematic Review and Meta-Analysis of Randomized Controlled Studies.J Clin Med. 2024 Feb 19;13(4):1174. doi: 10.3390/jcm13041174. J Clin Med. 2024. PMID: 38398486 Free PMC article. Review.
-
Prophylactic midazolam and clonidine for emergence from agitation in children after emergence from sevoflurane anesthesia: a meta-analysis.Clin Ther. 2013 Oct;35(10):1622-31. doi: 10.1016/j.clinthera.2013.08.016. Epub 2013 Sep 25. Clin Ther. 2013. PMID: 24075150 Review.
References
-
- Rosembaum A., Kain Z.N., Larsson P., Lonnqvist P.A., Wolf A.R. The place of premedication in pediatric practice. Pediatr. Anesth. 2009;19:817–828. - PubMed
-
- Fortier M., Martin S., Chorney J., Mayes L., Kain Z. Preoperative anxiety in adolescents undergoing surgery: A pilot study. Paediatric Anaesth. 2011;21:969–973. - PubMed
LinkOut - more resources
Full Text Sources