LDH-Based Voltammetric Sensors
- PMID: 38793212
- PMCID: PMC11123164
- DOI: 10.3390/mi15050640
LDH-Based Voltammetric Sensors
Abstract
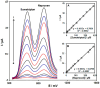
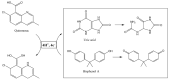
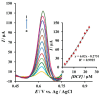
Layered double hydroxides (LDHs), also named hydrotalcite-like compounds, are anionic clays with a lamellar structure which have been extensively used in the last two decades as electrode modifiers for the design of electrochemical sensors. These materials can be classified into LDHs containing or not containing redox-active centers. In the former case, a transition metal cation undergoing a reversible redox reaction within a proper potential window is present in the layers, and, therefore, it can act as electron transfer mediator, and electrocatalyze the oxidation of an analyte for which the required overpotential is too high. In the latter case, a negatively charged species acting as a redox mediator can be introduced into the interlayer spaces after exchanging the anion coming from the synthesis, and, again, the material can display electrocatalytic properties. Alternatively, due to the large specific surface area of LDHs, molecules with electroactivity can be adsorbed on their surface. In this review, the most significant electroanalytical applications of LDHs as electrode modifiers for the development of voltammetric sensors are presented, grouping them based on the two types of materials.
Keywords: anion exchangeability; electrocatalysis; layered double hydroxides; modified electrodes; voltammetric sensors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Wang Y., Liang Y., Zhang S., Wang T., Zhuang A., Tian C., Luan F., Ni S.-Q., Fu X. Enhanced electrochemical sensor based on gold nanoparticles and MoS2 nanoflowers decorated ionic liquid-functionalized graphene for sensitive detection of bisphenol A in environmental water. Microchem. J. 2021;161:105769. doi: 10.1016/j.microc.2020.105769. - DOI
-
- Vlamidis Y., Fiorilli S., Giorgetti M., Gualandi I., Scavetta E., Tonelli D. Role of Fe in the oxidation of methanol electrocatalyzed by Ni based layered double hydroxides: X-ray spectroscopic and electrochemical studies. RSC Adv. 2016;6:110976. doi: 10.1039/c6ra19192d. - DOI
-
- Amini R., Asadpour-Zeynal K. Layered double hydroxide decorated with Ag nanodendrites as an enhanced sensing platform for voltammetric determination of pyrazinamide. New J. Chem. 2018;42:2140. doi: 10.1039/c7nj04544a. - DOI
Publication types
LinkOut - more resources
Full Text Sources

