COVID-19 Variants and Vaccine Development
- PMID: 38793638
- PMCID: PMC11125726
- DOI: 10.3390/v16050757
COVID-19 Variants and Vaccine Development
Abstract
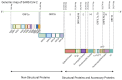
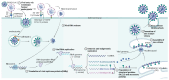
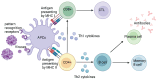
Coronavirus disease 2019 (COVID-19), the global pandemic caused by severe acute respiratory syndrome 2 virus (SARS-CoV-2) infection, has caused millions of infections and fatalities worldwide. Extensive SARS-CoV-2 research has been conducted to develop therapeutic drugs and prophylactic vaccines, and even though some drugs have been approved to treat SARS-CoV-2 infection, treatment efficacy remains limited. Therefore, preventive vaccination has been implemented on a global scale and represents the primary approach to combat the COVID-19 pandemic. Approved vaccines vary in composition, although vaccine design has been based on either the key viral structural (spike) protein or viral components carrying this protein. Therefore, mutations of the virus, particularly mutations in the S protein, severely compromise the effectiveness of current vaccines and the ability to control COVID-19 infection. This review begins by describing the SARS-CoV-2 viral composition, the mechanism of infection, the role of angiotensin-converting enzyme 2, the host defence responses against infection and the most common vaccine designs. Next, this review summarizes the common mutations of SARS-CoV-2 and how these mutations change viral properties, confer immune escape and influence vaccine efficacy. Finally, this review discusses global strategies that have been employed to mitigate the decreases in vaccine efficacy encountered against new variants.
Keywords: COVID-19; SARS-CoV-2; mutations; vaccination; vaccine effectiveness; variants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19) StatPearls Publishing; Treasure Island, FL, USA: 2023. - PubMed
-
- Liu D.X., Liang J.Q., Fung T.S. Human coronavirus-229E,-OC43,-NL63, and-HKU1 (Coronaviridae) Encycl. Virol. 2021;2:428–440. doi: 10.1016/B978-0-12-809633-8.21501-X. - DOI
-
- Brief W.S. Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions. World Health Organization; Geneva, Switzerland: 2020.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

