Advax-SM™-Adjuvanted COBRA (H1/H3) Hemagglutinin Influenza Vaccines
- PMID: 38793706
- PMCID: PMC11125990
- DOI: 10.3390/vaccines12050455
Advax-SM™-Adjuvanted COBRA (H1/H3) Hemagglutinin Influenza Vaccines
Abstract
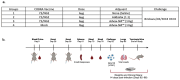
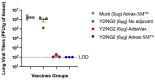
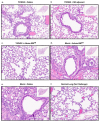
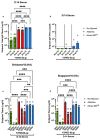
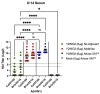
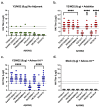
Adjuvants enhance immune responses stimulated by vaccines. To date, many seasonal influenza vaccines are not formulated with an adjuvant. In the present study, the adjuvant Advax-SM™ was combined with next generation, broadly reactive influenza hemagglutinin (HA) vaccines that were designed using a computationally optimized broadly reactive antigen (COBRA) methodology. Advax-SM™ is a novel adjuvant comprising inulin polysaccharide and CpG55.2, a TLR9 agonist. COBRA HA vaccines were combined with Advax-SM™ or a comparator squalene emulsion (SE) adjuvant and administered to mice intramuscularly. Mice vaccinated with Advax-SM™ adjuvanted COBRA HA vaccines had increased serum levels of anti-influenza IgG and IgA, high hemagglutination inhibition activity against a panel of H1N1 and H3N2 influenza viruses, and increased anti-influenza antibody secreting cells isolated from spleens. COBRA HA plus Advax-SM™ immunized mice were protected against both morbidity and mortality following viral challenge and, at postmortem, had no detectable lung viral titers or lung inflammation. Overall, the Advax-SM™-adjuvanted COBRA HA formulation provided effective protection against drifted H1N1 and H3N2 influenza viruses.
Keywords: Advax-SM™; COBRA; adjuvant; influenza; vaccine.
Conflict of interest statement
P.S. and T.M.R. declare no competing interests. G.A., A.A. and N.P. are all affiliated with Vaxine Pty Ltd., which holds proprietary rights over Advax®, CpG55.2™, and Advax-SM™ adjuvants.
Figures


References
-
- Paget J., Spreeuwenberg P., Charu V., Taylor R.J., Iuliano A.D., Bresee J., Simonsen L., Viboud C., Global Seasonal Influenza-Associated Mortality Collaborator Network and GLaMOR Collaborating Teams Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health. 2019;9:020421. doi: 10.7189/jogh.09.020421. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

