The D Gene in CDR H3 Determines a Public Class of Human Antibodies to SARS-CoV-2
- PMID: 38793718
- PMCID: PMC11126049
- DOI: 10.3390/vaccines12050467
The D Gene in CDR H3 Determines a Public Class of Human Antibodies to SARS-CoV-2
Abstract
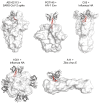
Public antibody responses have been found against many infectious agents. Structural convergence of public antibodies is usually determined by immunoglobulin V genes. Recently, a human antibody public class against SARS-CoV-2 was reported, where the D gene (IGHD3-22) encodes a common YYDxxG motif in heavy-chain complementarity-determining region 3 (CDR H3), which determines specificity for the receptor-binding domain (RBD). In this review, we discuss the isolation, structural characterization, and genetic analyses of this class of antibodies, which have been isolated from various cohorts of COVID-19 convalescents and vaccinees. All eleven YYDxxG antibodies with available structures target the SARS-CoV-2 RBD in a similar binding mode, where the CDR H3 dominates the interaction with antigen. The antibodies target a conserved site on the RBD that does not overlap with the receptor-binding site, but their particular angle of approach results in direct steric hindrance to receptor binding, which enables both neutralization potency and breadth. We also review the properties of CDR H3-dominant antibodies that target other human viruses. Overall, unlike most public antibodies, which are identified by their V gene usage, this newly discovered public class of YYDxxG antibodies is dominated by a D-gene-encoded motif and uncovers further opportunities for germline-targeting vaccine design.
Keywords: CDR H3; D gene; SARS-CoV-2; broadly neutralizing antibody; public antibody.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Fiolet T., Kherabi Y., MacDonald C.J., Ghosn J., Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022;28:202–221. doi: 10.1016/j.cmi.2021.10.005. - DOI - PMC - PubMed
-
- Barnes C.O., West A.P., Jr., Huey-Tubman K.E., Hoffmann M.A.G., Sharaf N.G., Hoffman P.R., Koranda N., Gristick H.B., Gaebler C., Muecksch F., et al. Structures of human antibodies bound to SARS-CoV-2 spike reveal common epitopes and recurrent features of antibodies. Cell. 2020;182:828–842.e16. doi: 10.1016/j.cell.2020.06.025. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

