Immunogenicity and Safety of SARS-CoV-2 Protein Subunit Recombinant Vaccine (IndoVac®) as a Booster Dose against COVID-19 in Indonesian Adults
- PMID: 38793791
- PMCID: PMC11125677
- DOI: 10.3390/vaccines12050540
Immunogenicity and Safety of SARS-CoV-2 Protein Subunit Recombinant Vaccine (IndoVac®) as a Booster Dose against COVID-19 in Indonesian Adults
Abstract
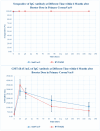
According to the WHO target product profile for COVID-19 vaccines, the vaccine in development should be indicated for active immunisation in all populations. Therefore, PT Bio Farma developed a candidate vaccine in a subunit protein recombinant platform to help overcome the issue. This trial was an observer-blind, randomised, prospective intervention study. This study targeted individuals who had received complete primary doses of the authorised/approved COVID-19 vaccine. The groups were divided into the primary inactivated vaccine (CoronaVac®) group, the primary viral vector vaccine (ChAdOx1) group, and the primary mRNA vaccine (BNT162b2) group that received the recombinant protein (IndoVac®). The groups were compared with the control and primary mRNA vaccine (BNT162b2). The participants enrolled in the study were from two primary care centres in Bandung City and three primary care centres in Denpasar City. A total of 696 participants were enrolled from 1 September to 31 October 2022. The demographic characteristics of the all-vaccine group showed a uniform distribution. The results showed that, compared with the control, the investigational product had inferior effectiveness 14 days after the booster dose was administered. However, 28 days after the booster dose, the investigational product exhibited non-inferior effectiveness compared with the primary groups that received CoronaVac® (GMR 0.76 (0.57-0.99)) and ChAdOx1 (GMR 0.72 (0.56-59.93)), but the BNT162b2 group (GMR 0.61 (0.39-0.94)) was inferior to the control. At 12 months follow-up after the booster dose, three serious adverse events (SAEs) were reported in three participants, with causality not correlated with the investigated products. Neither AEs of special interest nor severe COVID-19 cases were reported throughout the follow-up period; thus, the IndoVac® vaccine as a booster was immunogenic and safe. Until the 6-month follow-up after the booster dose, the IndoVac® vaccine was well tolerated and all reported AEs resolved. This vaccine is registered and can be included in the immunisation programme.
Keywords: COVID-19; immunogenicity; protein recombinant vaccine (IndoVac®); safety.
Conflict of interest statement
Ulfa Luthfiani Nurkamila and Mita Puspita were employees of PT Bio Farma at the time of conducting this study and preparing the manuscript.
Figures






References
-
- Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979. doi: 10.1016/S0140-6736(20)32466-1. - DOI - PMC - PubMed
-
- Hernández-Bernal F., Ricardo-Cobas M.C., Martín-Bauta Y., Rodríguez-Martínez E., Urrutia-Pérez K., Urrutia-Pérez K., Quintana-Guerra J., Navarro-Rodríguez Z., Piñera-Martínez M., Rodríguez-Reinoso J.L., et al. A phase 3, randomised, double-blind, placebo-controlled clinical trial evaluation of the efficacy and safety of a SARS-CoV-2 recombinant spike RBD protein vaccine in adults (ABDALA-3 study) Lancet Reg. Health Am. 2023;21:100497. doi: 10.1016/j.lana.2023.100497. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

