A Low Number of Baselines γδ T Cells Increases the Risk of SARS-CoV-2 Post-Vaccination Infection
- PMID: 38793803
- PMCID: PMC11125751
- DOI: 10.3390/vaccines12050553
A Low Number of Baselines γδ T Cells Increases the Risk of SARS-CoV-2 Post-Vaccination Infection
Abstract
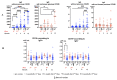
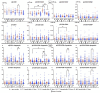
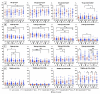
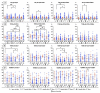
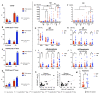
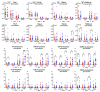
Background: The COVID-19 pandemic is the biggest global health problem in the last hundred years. The efficacy of the vaccine to protect against severe disease is estimated to be 70-95% according to the studies carried out, although there are aspects of the immune response to the vaccine that remain unclear. Methods: Humoral and cellular immunity after the administration of three doses of the Pfizer-BioNTech and Oxford AstraZeneca vaccines against SARS-CoV-2 over one year and the appearance of post-vaccination COVID-19 were studied. SARS-CoV-2 IgG and IgA antibodies, αβ and γδ T-cell subsets, and their differentiation stages and apoptosis were analyzed. Results: Anti-SARS-CoV-2 IgG and IgA antibodies showed a progressive increase throughout the duration of the study. This increase was the greatest after the third dose. The highest levels were observed in subjects who had anti-SARS-CoV-2 antibodies prior to vaccination. There was an increase in CD4+ αβ, CD8+ γδ and TEM CD8+ γδ T cells, and a decrease in apoptosis in CD4+ CD8+ and CD56+ αβ and γδ T cells. Post-vaccination SARS-CoV-2 infection was greater than 60%. The symptoms of COVID-19 were very mild and were related to a γδ T cell deficit, specifically CD8+ TEMRA and CD56+ γδ TEM, as well as lower pre-vaccine apoptosis levels. Conclusions: The results unveil the important role of γδ T cells in SARS-CoV-2-vaccine-mediated protection from the disease.
Keywords: SARS-CoV-2; antibodies; vaccine; αβ T cells; γδ T cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Cell immunity to SARS-CoV-2 after natural infection and/or different vaccination regimens.Front Cell Infect Microbiol. 2024 Mar 20;14:1370859. doi: 10.3389/fcimb.2024.1370859. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38572317 Free PMC article.
-
Robust Vaccine-Induced as Well as Hybrid B- and T-Cell Immunity across SARS-CoV-2 Vaccine Platforms in People with HIV.Microbiol Spectr. 2023 Jun 15;11(3):e0115523. doi: 10.1128/spectrum.01155-23. Epub 2023 May 11. Microbiol Spectr. 2023. PMID: 37166335 Free PMC article.
-
Immune Response to SARS-CoV-2 Vaccine in 2 Men.Int Arch Allergy Immunol. 2022;183(3):350-359. doi: 10.1159/000520046. Epub 2021 Nov 18. Int Arch Allergy Immunol. 2022. PMID: 34794147 Free PMC article.
-
[Humoral immunity against SARS-CoV-2 in workers of social health care centers of Castilla y León after vaccination with the BNT162b2 mRNA vaccine from Pfizer/Biontech.].Rev Esp Salud Publica. 2021 Oct 25;95:e202110141. Rev Esp Salud Publica. 2021. PMID: 34690347 Spanish.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
References
-
- Gutfreund M.C., Kobayashi T., Callado G.Y., Pardo I., Hsieh M.K., Lin V., Perencevich E.N., Salinas J.L., Edmond M.B., Mendonça E., et al. The effectiveness of the COVID-19 vaccines in the prevention of post-COVID conditions in children and adolescents: A systematic literature review and meta-analysis. Antimicrob. Steward. Healthc. Epidemiol. 2024;4:e54. doi: 10.1017/ash.2024.42. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

