SARS-CoV-2 Neutralization Assays Used in Clinical Trials: A Narrative Review
- PMID: 38793805
- PMCID: PMC11125816
- DOI: 10.3390/vaccines12050554
SARS-CoV-2 Neutralization Assays Used in Clinical Trials: A Narrative Review
Abstract
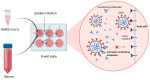
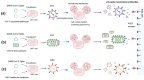
Since the emergence of COVID-19, extensive research efforts have been undertaken to accelerate the development of multiple types of vaccines to combat the pandemic. These include inactivated, recombinant subunit, viral vector, and nucleic acid vaccines. In the development of these diverse vaccines, appropriate methods to assess vaccine immunogenicity are essential in both preclinical and clinical studies. Among the biomarkers used in vaccine evaluation, the neutralizing antibody level serves as a pivotal indicator for assessing vaccine efficacy. Neutralizing antibody detection methods can mainly be classified into three types: the conventional virus neutralization test, pseudovirus neutralization test, and surrogate virus neutralization test. Importantly, standardization of these assays is critical for their application to yield results that are comparable across different laboratories. The development and use of international or regional standards would facilitate assay standardization and facilitate comparisons of the immune responses induced by different vaccines. In this comprehensive review, we discuss the principles, advantages, limitations, and application of different SARS-CoV-2 neutralization assays in vaccine clinical trials. This will provide guidance for the development and evaluation of COVID-19 vaccines.
Keywords: SARS-CoV-2; clinical trials; correlation of protection; neutralizing antibodies; standards.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Quantifying Absolute Neutralization Titers against SARS-CoV-2 by a Standardized Virus Neutralization Assay Allows for Cross-Cohort Comparisons of COVID-19 Sera.mBio. 2021 Feb 16;12(1):e02492-20. doi: 10.1128/mBio.02492-20. mBio. 2021. PMID: 33593976 Free PMC article.
-
Quantitative and Standardized Pseudovirus Neutralization Assay for COVID-19.Methods Mol Biol. 2024;2779:259-271. doi: 10.1007/978-1-0716-3738-8_11. Methods Mol Biol. 2024. PMID: 38526789
-
Immunogenicity of an adenovirus-vectored bivalent vaccine against wild type SARS-CoV-2 and Omicron variants in a murine model.Vaccine. 2024 Feb 27;42(6):1292-1299. doi: 10.1016/j.vaccine.2024.01.073. Epub 2024 Jan 30. Vaccine. 2024. PMID: 38296705
-
COVID-19 Pandemic and Vaccines Update on Challenges and Resolutions.Front Cell Infect Microbiol. 2021 Sep 10;11:690621. doi: 10.3389/fcimb.2021.690621. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34568087 Free PMC article. Review.
-
Overview of Neutralization Assays and International Standard for Detecting SARS-CoV-2 Neutralizing Antibody.Viruses. 2022 Jul 18;14(7):1560. doi: 10.3390/v14071560. Viruses. 2022. PMID: 35891540 Free PMC article. Review.
Cited by
-
A Meta-Analysis on the Immunogenicity of Homologous versus Heterologous Immunization Regimens against SARS-CoV-2 Beta, Delta, and Omicron BA.1 VoCs in Healthy Adults.J Microbiol Biotechnol. 2025 Feb 24;35:e2411059. doi: 10.4014/jmb.2411.11059. J Microbiol Biotechnol. 2025. PMID: 40147926 Free PMC article.
-
Advances in Surrogate Neutralization Tests for High-Throughput Screening and the Point-of-Care.Anal Chem. 2025 Mar 18;97(10):5407-5423. doi: 10.1021/acs.analchem.5c00666. Epub 2025 Mar 4. Anal Chem. 2025. PMID: 40035475 Free PMC article. Review. No abstract available.
-
A Live-Cell Imaging-Based Fluorescent SARS-CoV-2 Neutralization Assay by Antibody-Mediated Blockage of Receptor Binding Domain-ACE2 Interaction.BioTech (Basel). 2025 Feb 14;14(1):10. doi: 10.3390/biotech14010010. BioTech (Basel). 2025. PMID: 39982277 Free PMC article.
-
Centenarians, semi and supercentenarians, COVID-19 and Spanish flu: a serological assessment to gain insight into the resilience of older centenarians to COVID-19.Immun Ageing. 2024 Jun 27;21(1):44. doi: 10.1186/s12979-024-00450-3. Immun Ageing. 2024. PMID: 38937774 Free PMC article.
-
Recurrent waning of anti-SARS-CoV-2 neutralizing antibodies despite multiple antigen encounters.J Transl Med. 2025 Jul 11;23(1):783. doi: 10.1186/s12967-025-06837-0. J Transl Med. 2025. PMID: 40646552 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

