The Potential of Fecal Volatile Organic Compound Analysis for the Early Diagnosis of Late-Onset Sepsis in Preterm Infants: A Narrative Review
- PMID: 38794014
- PMCID: PMC11124895
- DOI: 10.3390/s24103162
The Potential of Fecal Volatile Organic Compound Analysis for the Early Diagnosis of Late-Onset Sepsis in Preterm Infants: A Narrative Review
Abstract
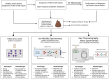
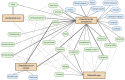
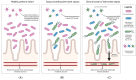
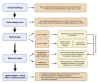
Early diagnosis and treatment of late-onset sepsis (LOS) is crucial for survival, but challenging. Intestinal microbiota and metabolome alterations precede the clinical onset of LOS, and the preterm gut is considered an important source of bacterial pathogens. Fecal volatile organic compounds (VOCs), formed by physiologic and pathophysiologic metabolic processes in the preterm gut, reflect a complex interplay between the human host, the environment, and microbiota. Disease-associated fecal VOCs can be detected with an array of devices with various potential for the development of a point-of-care test (POCT) for preclinical LOS detection. While characteristic VOCs for common LOS pathogens have been described, their VOC profiles often overlap with other pathogens due to similarities in metabolic pathways, hampering the construction of species-specific profiles. Clinical studies have, however, successfully discriminated LOS patients from healthy individuals using fecal VOC analysis with the highest predictive value for Gram-negative pathogens. This review discusses the current advancements in the development of a non-invasive fecal VOC-based POCT for early diagnosis of LOS, which may potentially provide opportunities for early intervention and targeted treatment and could improve clinical neonatal outcomes. Identification of confounding variables impacting VOC synthesis, selection of an optimal detection device, and development of standardized sampling protocols will allow for the development of a novel POCT in the near future.
Keywords: biomarker; detection; electronic nose; intestinal microbiota; late-onset sepsis; neonatology; non-invasive diagnostics; preterm infants; volatile organic compounds.
Conflict of interest statement
N.M.F., H.J.N. and T.G.J.d.M. have received an unrestricted grant from Nutricia Benelux Corporation outside this submitted work. N.K.d.B. has served as a speaker for AbbVie and MSD and has served as a consultant and principal investigator for TEVA Pharma BV and Takeda. He has received a research grant (unrestricted) from Dr. Falk, TEVA Pharma BV, Dutch Digestive Foundation (MLDS), and Takeda: all outside the submitted work.
Figures
References
-
- Villavicencio F., Perin J., Eilerts-Spinelli H., Yeung D., Prieto-Merino D., Hug L., Sharrow D., You D., Strong K.L., Black R.E., et al. Global, regional, and national causes of death in children and adolescents younger than 20 years: An open data portal with estimates for 2000–2021. Lancet Glob. Health. 2024;12:e16–e17. doi: 10.1016/S2214-109X(23)00496-5. - DOI - PubMed
-
- Manuck T.A., Rice M.M., Bailit J.L., Grobman W.A., Reddy U.M., Wapner R.J., Thorp J.M., Caritis S.N., Prasad M., Tita A.T., et al. Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. Am. J. Obstet. Gynecol. 2016;215:103.e101–103.e114. doi: 10.1016/j.ajog.2016.01.004. - DOI - PMC - PubMed
-
- Letouzey M., Foix-L’Hélias L., Torchin H., Mitha A., Morgan A.S., Zeitlin J., Kayem G., Maisonneuve E., Delorme P., Khoshnood B., et al. Cause of preterm birth and late-onset sepsis in very preterm infants: The EPIPAGE-2 cohort study. Pediatr. Res. 2021;90:584–592. doi: 10.1038/s41390-021-01411-y. - DOI - PMC - PubMed
-
- Tröger B., Göpel W., Faust K., Müller T., Jorch G., Felderhoff-Müser U., Gortner L., Heitmann F., Hoehn T., Kribs A., et al. Risk for late-onset blood-culture proven sepsis in very-low-birth weight infants born small for gestational age: A large multicenter study from the German Neonatal Network. Pediatr. Infect. Dis. J. 2014;33:238–243. doi: 10.1097/inf.0000000000000031. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

