Shape Matters: Impact of Mesoporous Silica Nanoparticle Morphology on Anti-Tumor Efficacy
- PMID: 38794294
- PMCID: PMC11125244
- DOI: 10.3390/pharmaceutics16050632
Shape Matters: Impact of Mesoporous Silica Nanoparticle Morphology on Anti-Tumor Efficacy
Abstract
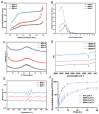
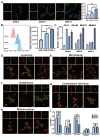
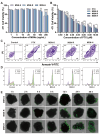
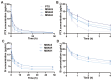
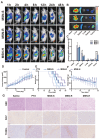
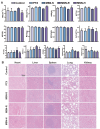
A nanoparticle's shape is a critical determinant of its biological interactions and therapeutic effectiveness. This study investigates the influence of shape on the performance of mesoporous silica nanoparticles (MSNs) in anticancer therapy. MSNs with spherical, rod-like, and hexagonal-plate-like shapes were synthesized, with particle sizes of around 240 nm, and their other surface properties were characterized. The drug loading capacities of the three shapes were controlled to be 47.46%, 49.41%, and 46.65%, respectively. The effects of shape on the release behaviors, cellular uptake mechanisms, and pharmacological behaviors of MSNs were systematically investigated. Through a series of in vitro studies using 4T1 cells and in vivo evaluations in 4T1 tumor-bearing mice, the release kinetics, cellular behaviors, pharmacological effects, circulation profiles, and therapeutic efficacy of MSNs were comprehensively assessed. Notably, hexagonal-plate-shaped MSNs loaded with PTX exhibited a prolonged circulation time (t1/2 = 13.59 ± 0.96 h), which was approximately 1.3 times that of spherical MSNs (t1/2 = 10.16 ± 0.38 h) and 1.5 times that of rod-shaped MSNs (t1/2 = 8.76 ± 1.37 h). This research underscores the significance of nanoparticles' shapes in dictating their biological interactions and therapeutic outcomes, providing valuable insights for the rational design of targeted drug delivery systems in cancer therapy.
Keywords: anticancer therapy; biodistribution; drug delivery; mesoporous silica nanoparticles (MSNs); shape effect.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

