Topotecan and Ginkgolic Acid Inhibit the Expression and Transport Activity of Human Organic Anion Transporter 3 by Suppressing SUMOylation of the Transporter
- PMID: 38794300
- PMCID: PMC11124914
- DOI: 10.3390/pharmaceutics16050638
Topotecan and Ginkgolic Acid Inhibit the Expression and Transport Activity of Human Organic Anion Transporter 3 by Suppressing SUMOylation of the Transporter
Abstract
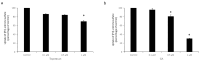
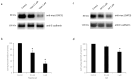
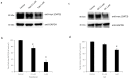
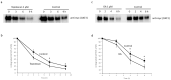
Organic anion transporter 3 (OAT3), expressed at the basolateral membrane of kidney proximal tubule cells, facilitates the elimination of numerous metabolites, environmental toxins, and clinically important drugs. An earlier investigation from our laboratory revealed that OAT3 expression and transport activity can be upregulated by SUMOylation, a post-translational modification that covalently conjugates SUMO molecules to substrate proteins. Topotecan is a semi-synthetic derivative of the herbal extract camptothecin, approved by the FDA to treat several types of cancer. Ginkgolic acid (GA) is one of the major components in the extract of Ginkgo biloba leaves that has long been used in food supplements for preventing dementia, high blood pressure, and supporting stroke recovery. Both topotecan and GA have been shown to affect protein SUMOylation. In the current study, we tested our hypothesis that topotecan and GA may regulate OAT3 SUMOylation, expression, and transport function. Our data show that the treatment of OAT3-expressing cells with topotecan or GA significantly decreases the SUMOylation of OAT3 by 50% and 75%, respectively. The same treatment also led to substantial reductions in OAT3 expression and the OAT3-mediated transport of estrone sulfate, a prototypical substrate. Such reductions in cell surface expression of OAT3 correlated well with an increased rate of OAT3 degradation. Mechanistically, we discovered that topotecan enhanced the association between OAT3 and the SUMO-specific protease SENP2, a deSUMOylation enzyme, which contributed to the significant decrease in OAT3 SUMOylation. In conclusion, this study unveiled a novel role of topotecan and GA in inhibiting OAT3 expression and transport activity and accelerating OAT3 degradation by suppressing OAT3 SUMOylation. During comorbidity therapies, the use of topotecan or Ginkgo biloba extract could potentially decrease the transport activity of OAT3 in the kidneys, which will in turn affect the therapeutic efficacy and toxicity of many other drugs that are substrates for the transporter.
Keywords: SUMOylation; drug transporter; organic anion transporter 3; post-translational modification; regulation; topotecan.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


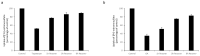



Similar articles
-
The SUMO-Specific Protease Senp2 Regulates SUMOylation, Expression and Function of Human Organic Anion Transporter 3.Biochim Biophys Acta Biomembr. 2019 Jul 1;1861(7):1293-1301. doi: 10.1016/j.bbamem.2019.04.007. Epub 2019 May 1. Biochim Biophys Acta Biomembr. 2019. PMID: 31054272 Free PMC article.
-
Activation of Protein Kinase A Stimulates SUMOylation, Expression, and Transport Activity of Organic Anion Transporter 3.AAPS J. 2019 Feb 13;21(2):30. doi: 10.1208/s12248-019-0303-4. AAPS J. 2019. PMID: 30761470 Free PMC article.
-
Chloroquine and Hydroxychloroquine, as Proteasome Inhibitors, Upregulate the Expression and Activity of Organic Anion Transporter 3.Pharmaceutics. 2023 Jun 14;15(6):1725. doi: 10.3390/pharmaceutics15061725. Pharmaceutics. 2023. PMID: 37376173 Free PMC article.
-
The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules.Biochim Biophys Acta. 2003 Dec 30;1618(2):185-93. doi: 10.1016/j.bbamem.2003.08.015. Biochim Biophys Acta. 2003. PMID: 14729155 Review.
-
Transport of organic anions across the basolateral membrane of proximal tubule cells.Rev Physiol Biochem Pharmacol. 2003;146:95-158. doi: 10.1007/s10254-002-0003-8. Epub 2003 Jan 30. Rev Physiol Biochem Pharmacol. 2003. PMID: 12605306 Review.
Cited by
-
Recent Advances on the Regulations of Organic Anion Transporters.Pharmaceutics. 2024 Oct 24;16(11):1355. doi: 10.3390/pharmaceutics16111355. Pharmaceutics. 2024. PMID: 39598479 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

