Synthesis, Characterisation, and In Vitro Evaluation of Biocompatibility, Antibacterial and Antitumor Activity of Imidazolium Ionic Liquids
- PMID: 38794304
- PMCID: PMC11125126
- DOI: 10.3390/pharmaceutics16050642
Synthesis, Characterisation, and In Vitro Evaluation of Biocompatibility, Antibacterial and Antitumor Activity of Imidazolium Ionic Liquids
Abstract
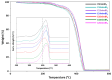
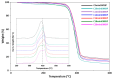
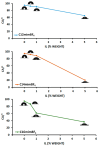
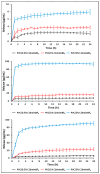
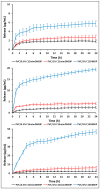
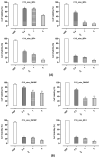
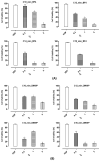
In recent decades, ionic liquids (ILs) have garnered research interest for their noteworthy properties, such as thermal stability, low or no flammability, and negligible vapour pressure. Moreover, their tunability offers limitless opportunities to design ILs with properties suitable for applications in many industrial fields. This study aims to synthetise two series of methylimidazolium ILs bearing long alkyl chain in their cations (C9, C10, C12, C14, C16, C18, C20) and with tetrafluoroborate (BF4) and the 1,3-dimethyl-5-sulfoisophthalate (DMSIP) as counter ions. The ILs were characterised using 1H-NMR and MALDI-TOF, and their thermal behaviour was investigated through DSC and TGA. Additionally, the antimicrobial, anticancer, and cytotoxic activities of the ILs were analysed. Moreover, the most promising ILs were incorporated at different concentrations (0.5, 1, 5 wt%) into polyvinyl chloride (PVC) by solvent casting to obtain antimicrobial blend films. The thermal properties and stability of the resulting PVC/IL films, along with their hydrophobicity/hydrophilicity, IL surface distribution, and release, were studied using DSC and TGA, contact angle (CA), SEM, and UV-vis spectrometry, respectively. Furthermore, the antimicrobial and cytotoxic properties of blends were analysed. The in vitro results demonstrated that the antimicrobial and antitumor activities of pure ILs against t Listeria monocytogenes, Escherichia coli, Pseudomonas fluorescens strains, and the breast cancer cell line (MCF7), respectively, were mainly dependent on their structure. These activities were higher in the series containing the BF4 anion and increased with the increase in the methylimidazolium cation alkyl chain length. However, the elongation of the alkyl chain beyond C16 induced a decrease in antimicrobial activity, indicating a cut-off effect. A similar trend was also observed in terms of in vitro biocompatibility. The loading of both the series of ILs into the PVC matrix did not affect the thermal stability of PVC blend films. However, their Tonset decreased with increased IL concentration and alkyl chain length. Similarly, both the series of PVC/IL films became more hydrophilic with increasing IL concentration and alkyl chain. The loading of ILs at 5% concentration led to considerable IL accumulation on the blend film surfaces (as observed in SEM images) and, subsequently, their higher release. The biocompatibility assessment with healthy human dermal fibroblast (HDF) cells and the investigation of antitumoral properties unveiled promising pharmacological characteristics. These findings provide strong support for the potential utilisation of ILs in biomedical applications, especially in the context of cancer therapy and as antibacterial agents to address the challenge of antibiotic resistance. Furthermore, the unique properties of the PVC/IL films make them versatile materials for advancing healthcare technologies, from drug delivery to tissue engineering and antimicrobial coatings to diagnostic devices.
Keywords: PVC/IL films; antibacterial tests; antitumor activity; in vitro cytotoxicity; methylimidazolium-based ionic liquids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Spellberg B., Guidos R., Gilbert J.B., Boucher H.W., Scheld M., Bartlett J.G., Edwards J. The Epidemic of Antibiotic-Resistant Infections: A Call to Action for the Medical Community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2008;46:155–164. doi: 10.1086/524891. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

