Nanodrug Delivery Systems for Myasthenia Gravis: Advances and Perspectives
- PMID: 38794313
- PMCID: PMC11125447
- DOI: 10.3390/pharmaceutics16050651
Nanodrug Delivery Systems for Myasthenia Gravis: Advances and Perspectives
Abstract
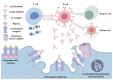
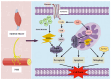
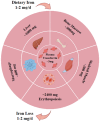
Myasthenia gravis (MG) is a rare chronic autoimmune disease caused by the production of autoantibodies against the postsynaptic membrane receptors present at the neuromuscular junction. This condition is characterized by fatigue and muscle weakness, including diplopia, ptosis, and systemic impairment. Emerging evidence suggests that in addition to immune dysregulation, the pathogenesis of MG may involve mitochondrial damage and ferroptosis. Mitochondria are the primary site of energy production, and the reactive oxygen species (ROS) generated due to mitochondrial dysfunction can induce ferroptosis. Nanomedicines have been extensively employed to treat various disorders due to their modifiability and good biocompatibility, but their application in MG management has been rather limited. Nevertheless, nanodrug delivery systems that carry immunomodulatory agents, anti-oxidants, or ferroptosis inhibitors could be effective for the treatment of MG. Therefore, this review focuses on various nanoplatforms aimed at attenuating immune dysregulation, restoring mitochondrial function, and inhibiting ferroptosis that could potentially serve as promising agents for targeted MG therapy.
Keywords: ferroptosis; immunomodulation; mitochondrial dysfunction; myasthenia gravis; nanomedicine.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
Similar articles
-
Myasthenia gravis.Dis Mon. 1997 Feb;43(2):65-109. doi: 10.1016/s0011-5029(97)90033-x. Dis Mon. 1997. PMID: 9118787 Review.
-
Update on Ocular Myasthenia Gravis.Neurol Clin. 2017 Feb;35(1):115-123. doi: 10.1016/j.ncl.2016.08.008. Neurol Clin. 2017. PMID: 27886889 Review.
-
Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice.Brain. 2012 Apr;135(Pt 4):1081-101. doi: 10.1093/brain/aws025. Epub 2012 Mar 6. Brain. 2012. PMID: 22396395
-
Myasthenia gravis. Update on diagnosis and therapy.Med Clin (Barc). 2023 Aug 11;161(3):119-127. doi: 10.1016/j.medcli.2023.04.006. Epub 2023 May 27. Med Clin (Barc). 2023. PMID: 37248131 Review. English, Spanish.
-
Ocular myasthenia gravis: a review and practical guide for clinicians.Clin Exp Optom. 2022 Mar;105(2):205-213. doi: 10.1080/08164622.2022.2029683. Epub 2022 Feb 14. Clin Exp Optom. 2022. PMID: 35157811 Review.
Cited by
-
Exploring the Potential of Mitochondria-Targeted Drug Delivery for Enhanced Breast Cancer Therapy.Int J Breast Cancer. 2025 Mar 3;2025:3013009. doi: 10.1155/ijbc/3013009. eCollection 2025. Int J Breast Cancer. 2025. PMID: 40224721 Free PMC article. Review.
-
Iron metabolism in rheumatic diseases.J Transl Autoimmun. 2025 Jan 4;10:100267. doi: 10.1016/j.jtauto.2025.100267. eCollection 2025 Jun. J Transl Autoimmun. 2025. PMID: 39867458 Free PMC article. Review.
-
Immune imbalance in Lupus Nephritis: The intersection of T-Cell and ferroptosis.Front Immunol. 2024 Dec 12;15:1520570. doi: 10.3389/fimmu.2024.1520570. eCollection 2024. Front Immunol. 2024. PMID: 39726588 Free PMC article. Review.
-
Iron homeostasis and ferroptosis in human diseases: mechanisms and therapeutic prospects.Signal Transduct Target Ther. 2024 Oct 14;9(1):271. doi: 10.1038/s41392-024-01969-z. Signal Transduct Target Ther. 2024. PMID: 39396974 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

