Effects of Nozzle Retraction Elimination on Spray Distribution in Middle-Posterior Turbinate Regions: A Comparative Study
- PMID: 38794345
- PMCID: PMC11124954
- DOI: 10.3390/pharmaceutics16050683
Effects of Nozzle Retraction Elimination on Spray Distribution in Middle-Posterior Turbinate Regions: A Comparative Study
Abstract
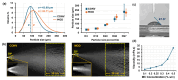
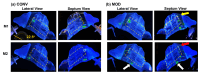
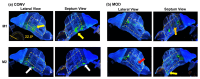
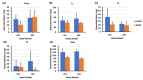
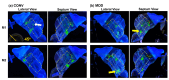
The standard multi-dose nasal spray pump features an integrated actuator and nozzle, which inevitably causes a retraction of the nozzle tip during application. The retraction stroke is around 5.5 mm and drastically reduces the nozzle's insertion depth, which further affects the initial nasal spray deposition and subsequent translocation, potentially increasing drug wastes and dosimetry variability. To address this issue, we designed a new spray pump that separated the nozzle from the actuator and connected them with a flexible tube, thereby eliminating nozzle retraction during application. The objective of this study is to test the new device's performance in comparison to the conventional nasal pump in terms of spray generation, plume development, and dosimetry distribution. For both devices, the spray droplet size distribution was measured using a laser diffraction particle analyzer. Plume development was recorded with a high-definition camera. Nasal dosimetry was characterized in two transparent nasal cavity casts (normal and decongested) under two breathing conditions (breath-holding and constant inhalation). The nasal formulation was a 0.25% w/v methyl cellulose aqueous solution with a fluorescent dye. For each test case, the temporospatial spray translocation in the nasal cavity was recorded, and the final delivered doses were quantified in five nasal regions. The results indicate minor differences in droplet size distribution between the two devices. The nasal plume from the new device presents a narrower plume angle. The head orientation, the depth at which the nozzle is inserted into the nostril, and the administration angle play crucial roles in determining the initial deposition of nasal sprays as well as the subsequent translocation of the liquid film/droplets. Quantitative measurements of deposition distributions in the nasal models were augmented with visualization recordings to evaluate the delivery enhancements introduced by the new device. With an extension tube, the modified device produced a lower spray output and delivered lower doses in the front, middle, and back turbinate than the conventional nasal pump. However, sprays from the new device were observed to penetrate deeper into the nasal passages, predominantly through the middle-upper meatus. This resulted in consistently enhanced dosing in the middle-upper turbinate regions while at the cost of higher drug loss to the pharynx.
Keywords: actuation; deposition distribution; high-speed imaging; liquid film translocation; nasal spray; nasal valve; particle size distribution; plume development; visualization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Burton M.J., Clarkson J.E., Goulao B., Glenny A.M., McBain A.J., Schilder A.G., Webster K.E., Worthington H.V. Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection. Cochrane Database Syst. Rev. 2020;9:Cd013626. - PMC - PubMed
-
- Williamson S., Dennison L., Greenwell K., Denison-Day J., Mowbray F., Richards-Hall S., Smith D., Bradbury K., Ainsworth B., Little P., et al. Using nasal sprays to prevent respiratory tract infections: A qualitative study of online consumer reviews and primary care patient interviews. BMJ Open. 2022;12:e059661. doi: 10.1136/bmjopen-2021-059661. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

