Comparison of Printable Biomaterials for Use in Neural Tissue Engineering: An In Vitro Characterization and In Vivo Biocompatibility Assessment
- PMID: 38794619
- PMCID: PMC11125121
- DOI: 10.3390/polym16101426
Comparison of Printable Biomaterials for Use in Neural Tissue Engineering: An In Vitro Characterization and In Vivo Biocompatibility Assessment
Abstract
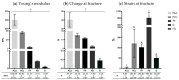
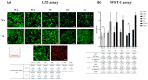
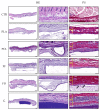
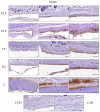
Nervous system traumatic injuries are prevalent in our society, with a significant socioeconomic impact. Due to the highly complex structure of the neural tissue, the treatment of these injuries is still a challenge. Recently, 3D printing has emerged as a promising alternative for producing biomimetic scaffolds, which can lead to the restoration of neural tissue function. The objective of this work was to compare different biomaterials for generating 3D-printed scaffolds for use in neural tissue engineering. For this purpose, four thermoplastic biomaterials, ((polylactic acid) (PLA), polycaprolactone (PCL), Filaflex (FF) (assessed here for the first time for biomedical purposes), and Flexdym (FD)) and gelatin methacrylate (GelMA) hydrogel were subjected to printability and mechanical tests, in vitro cell-biomaterial interaction analyses, and in vivo biocompatibility assessment. The thermoplastics showed superior printing results in terms of resolution and shape fidelity, whereas FD and GelMA revealed great viscoelastic properties. GelMA demonstrated a greater cell viability index after 7 days of in vitro cell culture. Moreover, all groups displayed connective tissue encapsulation, with some inflammatory cells around the scaffolds after 10 days of in vivo implantation. Future studies will determine the usefulness and in vivo therapeutic efficacy of novel neural substitutes based on the use of these 3D-printed scaffolds.
Keywords: 3D printing; biocompatibility; biomaterials; biomechanic; neural tissue engineering; scaffolds.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
Grants and funding
- FIS PI20/00318, PI23/00337/Ministry of Science and Innovation of Spain (Instituto de Salud Carlos III)
- Grant CPP2021-009070/Ministerio de Ciencia e Innovación, Unión Europea, Agencia Estatal de Investigación, España
- PTQ2019-010731/Ayudas Torres Quevedo
- FPU21/ 06183/FPU Fellowship, Spanish Ministry of Universities
LinkOut - more resources
Full Text Sources

