Propionate and butyrate counteract renal damage and progression to chronic kidney disease
- PMID: 38794880
- PMCID: PMC11852269
- DOI: 10.1093/ndt/gfae118
Propionate and butyrate counteract renal damage and progression to chronic kidney disease
Abstract
Background: Short-chain fatty acids (SCFAs), mainly acetate, propionate and butyrate, are produced by gut microbiota through fermentation of complex carbohydrates that cannot be digested by the human host. They affect gut health and can contribute at the distal level to the pathophysiology of several diseases, including renal pathologies.
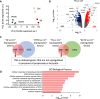
Methods: SCFA levels were measured in chronic kidney disease (CKD) patients (n = 54) at different stages of the disease, and associations with renal function and inflammation parameters were examined. The impact of propionate and butyrate in pathways triggered in tubular cells under inflammatory conditions was analysed using genome-wide expression assays. Finally, a pre-clinical mouse model of folic acid-induced transition from acute kidney injury to CKD was used to analyse the preventive and therapeutic potential of these microbial metabolites in the development of CKD.
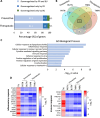
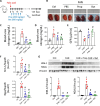
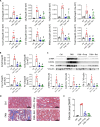
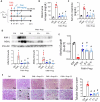
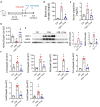
Results: Faecal levels of propionate and butyrate in CKD patients gradually reduce as the disease progresses, and do so in close association with established clinical parameters for serum creatinine, blood urea nitrogen and the estimated glomerular filtration rate. Propionate and butyrate jointly downregulated the expression of 103 genes related to inflammatory processes and immune system activation triggered by tumour necrosis factor-α in tubular cells. In vivo, the administration of propionate and butyrate, either before or soon after injury, respectively, prevented and slowed the progression of damage. This was indicated by a decrease in renal injury markers, the expression of pro-inflammatory and pro-fibrotic markers, and recovery of renal function over the long term.
Conclusions: Propionate and butyrate levels are associated with a progressive loss of renal function in CKD patients. Early administration of these SCFAs prevents disease advancement in a pre-clinical model of acute renal damage, demonstrating their therapeutic potential independently of the gut microbiota.
Keywords: AKI-to-CKD transition; acute kidney injury; fibrosis; inflammation; short-chain fatty acids.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




References
MeSH terms
Substances
Grants and funding
- Instituto de Salud Carlos III
- European Union
- IFEQ21/00203/Adquisición de equipamiento e Infraestructuras científico-técnicas
- RICORS2040 (RD21/0005/0017)/NextGenerationEU
- Mechanism for Recovery and Resilience (MRR)/RETC
- PCTI-Plan de Ciencia
- IDI/2021/000032/Tecnología e Innovación 2021-2023 del Gobierno del Principado de Asturias/FEDER
- Department of Industry, Tourism and Trade of the Government of the Autonomous Community of the Basque Country
- Innovation Technology Department of the Bizkaia County
- MINECO
- CEX2021-001136-S/Severo Ochoa Excellence Accreditation
- CP18/00106/The Miguel Servet Program from ISCIII
- FEDER-PCTI 2021-2023/Gobierno del Principado de Asturias
- Fundación para la Investigación Biosanitaria of Asturias
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

