Enrichment and characterization of human-associated mucin-degrading microbial consortia by sequential passage
- PMID: 38794902
- PMCID: PMC11180985
- DOI: 10.1093/femsec/fiae078
Enrichment and characterization of human-associated mucin-degrading microbial consortia by sequential passage
Abstract
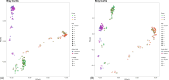
Mucin is a glycoprotein secreted throughout the mammalian gastrointestinal tract that can support endogenous microorganisms in the absence of complex polysaccharides. While several mucin-degrading bacteria have been identified, the interindividual differences in microbial communities capable of metabolizing this complex polymer are not well described. To determine whether community assembly on mucin is deterministic across individuals or whether taxonomically distinct but functionally similar mucin-degrading communities are selected across fecal inocula, we used a 10-day in vitro sequential batch culture fermentation from three human donors with mucin as the sole carbon source. For each donor, 16S rRNA gene amplicon sequencing was used to characterize microbial community succession, and the short-chain fatty acid profile was determined from the final community. All three communities reached a steady-state by day 7 in which the community composition stabilized. Taxonomic comparisons amongst communities revealed that one of the final communities had Desulfovibrio, another had Akkermansia, and all three shared other members, such as Bacteroides. Metabolic output differences were most notable for one of the donor's communities, with significantly less production of acetate and propionate than the other two communities. These findings demonstrate the feasibility of developing stable mucin-degrading communities with shared and unique taxa. Furthermore, the mechanisms and efficiencies of mucin degradation across individuals are important for understanding how this community-level process impacts human health.
Keywords: fermentation; gastrointestinal microbiome; microbial ecology; mucin.
© The Author(s) 2024. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
We have no known conflict of interest to disclose. This research has complied with all institutional and federal policies regarding the use of human subjects.
Figures

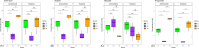


Similar articles
-
Rotavirus infection induces glycan availability to promote ileum-specific changes in the microbiome aiding rotavirus virulence.Gut Microbes. 2020 Sep 2;11(5):1324-1347. doi: 10.1080/19490976.2020.1754714. Epub 2020 May 13. Gut Microbes. 2020. PMID: 32404017 Free PMC article.
-
Effects of banana powder (Musa acuminata Colla) on the composition of human fecal microbiota and metabolic output using in vitro fermentation.J Food Sci. 2020 Aug;85(8):2554-2564. doi: 10.1111/1750-3841.15324. Epub 2020 Jul 16. J Food Sci. 2020. PMID: 32677055
-
The composition and metabolism of faecal microbiota is specifically modulated by different dietary polysaccharides and mucin: an isothermal microcalorimetry study.Benef Microbes. 2018 Jan 29;9(1):21-34. doi: 10.3920/BM2016.0198. Epub 2017 Oct 12. Benef Microbes. 2018. PMID: 29022389
-
Isolation and identification of mucin-degrading bacteria originated from human faeces and their potential probiotic efficacy according to host-microbiome enterotype.J Appl Microbiol. 2022 Aug;133(2):362-374. doi: 10.1111/jam.15560. Epub 2022 Apr 13. J Appl Microbiol. 2022. PMID: 35365862
-
Moving from genome-scale to community-scale metabolic models for the human gut microbiome.Nat Microbiol. 2025 May;10(5):1055-1066. doi: 10.1038/s41564-025-01972-2. Epub 2025 Apr 11. Nat Microbiol. 2025. PMID: 40217129 Review.
Cited by
-
The effect of dietary transition on infant microbiota composition and metabolic activity captured with the simulator of the human intestinal microbial ecosystem (SHIME).Gut Microbiome (Camb). 2025 Jun 13;6:e9. doi: 10.1017/gmb.2025.10007. eCollection 2025. Gut Microbiome (Camb). 2025. PMID: 40626280 Free PMC article.
-
Mechanism of 2'-fucosyllactose degradation by human-associated Akkermansia.J Bacteriol. 2024 Feb 22;206(2):e0033423. doi: 10.1128/jb.00334-23. Epub 2024 Feb 1. J Bacteriol. 2024. PMID: 38299857 Free PMC article.
-
A Pilot Study Exploring the Relationship Between Milk Composition and Microbial Capacity in Breastfed Infants.Nutrients. 2025 Jan 18;17(2):338. doi: 10.3390/nu17020338. Nutrients. 2025. PMID: 39861468 Free PMC article.
References
-
- Adamberg K, Kolk K, Jaagura M et al. The composition and metabolism of faecal microbiota is specifically modulated by different dietary polysaccharides and mucin: an isothermal microcalorimetry study. Benef Microbes. 2018;9:21–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

