Hypogammaglobulinaemia during rituximab treatment in multiple sclerosis: A Swedish cohort study
- PMID: 38794973
- PMCID: PMC11236063
- DOI: 10.1111/ene.16331
Hypogammaglobulinaemia during rituximab treatment in multiple sclerosis: A Swedish cohort study
Abstract
Background and purpose: Mechanisms behind hypogammaglobulinaemia during rituximab treatment are poorly understood.
Methods: In this register-based multi-centre retrospective cohort study of multiple sclerosis (MS) patients in Sweden, 2745 patients from six participating Swedish MS centres were identified via the Swedish MS registry and included between 14 March 2008 and 25 January 2021. The exposure was treatment with at least one dose of rituximab for MS or clinically isolated syndrome, including data on treatment duration and doses. The degree of yearly decrease in immunoglobulin G (IgG) and immunoglobulin M (IgM) levels was evaluated.
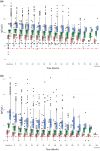
Results: The mean decrease in IgG was 0.27 (95% confidence interval 0.17-0.36) g/L per year on rituximab treatment, slightly less in older patients, and without significant difference between sexes. IgG or IgM below the lower limit of normal (<6.7 or <0.27 g/L) was observed in 8.8% and 8.3% of patients, respectively, as nadir measurements. Six out of 2745 patients (0.2%) developed severe hypogammaglobulinaemia (IgG below 4.0 g/L) during the study period. Time on rituximab and accumulated dose were the main predictors for IgG decrease. Previous treatment with fingolimod and natalizumab, but not teriflunomide, dimethyl fumarate, interferons or glatiramer acetate, were significantly associated with lower baseline IgG levels by 0.80-1.03 g/L, compared with treatment-naïve patients. Switching from dimethyl fumarate or interferons was associated with an additional IgG decline of 0.14-0.19 g/L per year, compared to untreated.
Conclusions: Accumulated dose and time on rituximab treatment are associated with a modest but significant decline in immunoglobulin levels. Previous MS therapies may influence additional IgG decline.
Keywords: IgG decrease; IgM decrease; disease‐modifying therapy; hypogammaglobulinaemia; immunoglobulin decrease; multiple sclerosis; real‐world data; rituximab therapy.
© 2024 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
SH, BE, EL, MB, PF, YW, JS, FN and AS reported no conflicts of interest. KF has received lecture honoraria and has served on scientific advisory boards for Biogen, Celgene, Janssen, Merck, Novartis and Roche. JL has received travel support and/or lecture honoraria and has served on scientific advisory boards for Alexion, Almirall, Biogen, Bristol Myers Squibb, Celgene, Janssen, Merck, Novartis, Roche and Sanofi; and has received unconditional research grants from Biogen and Novartis, and financial support from Sanofi for an investigator‐initiated study. TF has received funding from the ARTIS project, a national Swedish safety monitoring of rheumatology immunomodulators, in turn supported by agreements between Karolinska Institutet and Abbvie, BMS, Eli Lilly, Galapagos, MSD, Pfizer, Roche, Samsung Bioepis and Sanofi.
Figures

References
-
- Svenningsson A, Frisell T, Burman J, et al. Safety and efficacy of rituximab versus dimethyl fumarate in patients with relapsing–remitting multiple sclerosis or clinically isolated syndrome in Sweden: a rater‐blinded, phase 3, randomised controlled trial. Lancet Neurol. 2022;21(8):693‐703. doi:10.1016/S1474-4422(22)00209-5 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

