Effect of Avenciguat on Albuminuria in Patients with CKD: Two Randomized Placebo-Controlled Trials
- PMID: 38795055
- PMCID: PMC11387026
- DOI: 10.1681/ASN.0000000000000418
Effect of Avenciguat on Albuminuria in Patients with CKD: Two Randomized Placebo-Controlled Trials
Abstract
Key Points:
Despite new treatments for CKD, kidney failure risk remains high, particularly where albuminuria remains.
We report a prespecified pooled analysis of two randomized controlled trials assessing a soluble guanylate cyclase activator for CKD.
Avenciguat led to improvements in albuminuria in patients with CKD with/without type 2 diabetes mellitus, with acceptable safety.
Background:
Avenciguat is a novel, potent soluble guanylate cyclase activator in development for CKD. Two trials investigated avenciguat in diabetic (
Methods: A prespecified pooled analysis of two randomized, double-blind, placebo-controlled trials of identical design. Adults with CKD (eGFR ≥20 and <90 ml/min per 1.73 m2, urine albumin–creatinine ratio [UACR] ≥200 and <3500 mg/g) were randomized to 20 weeks of placebo or avenciguat 1, 2, or 3 mg three times daily (TID), adjunctive to angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. The primary end point was change from baseline in UACR in 10-hour urine at week 20, analyzed per protocol. The secondary end point was UACR change from baseline in first morning void urine at week 20. Safety was monitored throughout.
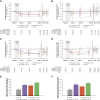
Results: Overall, 500 patients (mean age 62 years [SD 13]; mean eGFR 44 ml/min per 1.73 m2 [SD 18] and median 10-hour UACR 719 [interquartile range, 379–1285] mg/g) received placebo (n=122) or avenciguat 1 mg (n=125), 2 mg (n=126), or 3 mg (n=127) TID. All 243 patients in study one and 27 of 261 patients in study two had diabetes mellitus. Avenciguat 1, 2, and 3 mg TID reduced UACR in 10-hour and first morning void urine versus placebo throughout the treatment period. At week 20, placebo-corrected geometric mean changes (95% confidence interval) from baseline in UACR in 10-hour urine with avenciguat 1, 2, and 3 mg TID were −15.5% (−26.4 to −3.0), −13.2% (−24.6 to −0.1), and −21.5% (−31.7 to −9.8), respectively, analyzed per protocol. Corresponding changes in first morning void urine were −19.4% (−30.0 to −7.3), −15.5% (−26.9 to −2.5), and −23.4% (−33.5 to −11.8), respectively. Avenciguat was well tolerated; the overall frequency of adverse events was low and similar to placebo. The number of patients who discontinued the study drug because of adverse events with avenciguat 1, 2, and 3 mg TID were five (4%), 11 (9%), and 11 (9%), respectively, compared with four (3%) in the placebo group.
Conclusions: Avenciguat lowered albuminuria and was well tolerated in patients with CKD.
Clinical Trial registry name and registration number::
A Study to Test the Effect of Different Doses of BI 685509 on Kidney Function in People With Diabetic Kidney Disease,
Podcast:
This article contains a podcast at
Keywords: CKD; albuminuria; chronic diabetic complications; chronic kidney failure; chronic renal disease; clinical trial; creatinine; diabetes mellitus; diabetic kidney disease; randomized controlled trials.
Conflict of interest statement
Disclosure forms, as provided by each author, are available with the online version of the article at
Figures




Comment in
-
Avenciguat reduces albuminuria in patients with chronic kidney disease.Nat Rev Nephrol. 2024 Aug;20(8):493. doi: 10.1038/s41581-024-00866-6. Nat Rev Nephrol. 2024. PMID: 38956438 No abstract available.
References
-
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease; 2013. Accessed June 6, 2024. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

