A retrospective analysis of drugs associated with the development of cutaneous squamous cell carcinoma reported by patients on the FDA's adverse events reporting system
- PMID: 38795220
- PMCID: PMC11127877
- DOI: 10.1007/s00403-024-03109-7
A retrospective analysis of drugs associated with the development of cutaneous squamous cell carcinoma reported by patients on the FDA's adverse events reporting system
Abstract
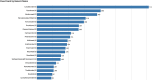
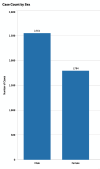
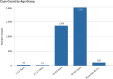
Cutaneous squamous cell carcinoma (cSCC) is the second most common type of skin cancer arising from squamous cells of the epidermis. Most cases of cSCC have a good prognosis if detected and treated early; however, certain cases can be aggressive. The primary risk factor for cSCC is prolonged ultraviolet radiation from sun exposure, leading to DNA mutations. Other risk factors have also been observed, including adverse reactions to medications, particularly immunosuppressants. A query of the Food and Drug Administration Adverse Events Reporting System (FAERS) was done, and all reported events of cSCC as adverse events to medication were recorded along with demographic data of patients affected. A total of 4,792 cases of cSCC as an adverse event to medication were reported between 1997 and 2023. Lenalidomide, a chemotherapeutic drug, had the most cases of cSCC as an adverse event. Nine of the top 10 drugs associated with cSCC had immunosuppressive characteristics. While males had higher odds of cSCC associated with corticosteroids and calcineurin inhibitors, females had higher odds of cSCC related to monoclonal antibodies. Geriatric patients accounted for the majority of cSCC cases at 59.7%. Drawing on data from the FAERS database, there's been a consistent increase in cSCC cases as a side-effect to certain medications, with most having immunosuppressive characteristics. Since there is a lack of up-to-date literature overviewing the most implicated medications for cSCC, we aimed to illustrate this better, as well as patient demographics, to better guide clinicians when prescribing these medications.
Keywords: Adverse events; Clinical research; Drugs; General dermatology; Medications; Side-effects; Skin cancer; Squamous cell carcinoma.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Occurrence of voriconazole-induced cutaneous squamous cell carcinoma in Japan: data mining from different national pharmacovigilance databases.Pharmazie. 2022 Oct 1;77(10):307-310. doi: 10.1691/ph.2022.2453. Pharmazie. 2022. PMID: 36273254
-
Association of Age, Sex, Race, and Geographic Region With Variation of the Ratio of Basal Cell to Cutaneous Squamous Cell Carcinomas in the United States.JAMA Dermatol. 2020 Nov 1;156(11):1192-1198. doi: 10.1001/jamadermatol.2020.2571. JAMA Dermatol. 2020. PMID: 32845319 Free PMC article.
-
Cutaneous squamous cell carcinoma (cSCC) and immunosurveillance - the impact of immunosuppression on frequency of cSCC.J Eur Acad Dermatol Venereol. 2019 Dec;33 Suppl 8:33-37. doi: 10.1111/jdv.16025. J Eur Acad Dermatol Venereol. 2019. PMID: 31833604 Review.
-
Immunosuppressive treatment after solid organ transplantation and risk of post-transplant cutaneous squamous cell carcinoma.Nephrol Dial Transplant. 2010 Aug;25(8):2764-71. doi: 10.1093/ndt/gfp425. Epub 2009 Sep 3. Nephrol Dial Transplant. 2010. PMID: 19729465
-
Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline.Eur J Cancer. 2015 Sep;51(14):1989-2007. doi: 10.1016/j.ejca.2015.06.110. Epub 2015 Jul 25. Eur J Cancer. 2015. PMID: 26219687 Review.
Cited by
-
A pharmacovigilance analysis of abrocitinib-related skin adverse events based on the FDA Adverse Event Reporting System (FAERS).Arch Dermatol Res. 2025 Feb 15;317(1):419. doi: 10.1007/s00403-025-03959-9. Arch Dermatol Res. 2025. PMID: 39954042
References
-
- Dreyfuss I, Kamath P, Frech F, Hernandez L, Nouri K (2022) Squamous cell carcinoma: 2021 updated review of treatment. Dermatol Ther 35(4). 10.1111/dth.15308 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

