A phase 1 study of concurrent cabozantinib and cetuximab in recurrent or metastatic head and neck squamous cell cancer
- PMID: 38795600
- PMCID: PMC11235871
- DOI: 10.1016/j.oraloncology.2024.106861
A phase 1 study of concurrent cabozantinib and cetuximab in recurrent or metastatic head and neck squamous cell cancer
Abstract
Objectives: Epidermal growth factor receptor (EGFR) inhibition with cetuximab is a standard treatment for head and neck squamous cell carcinoma (HNSCC). Activation of the receptor tyrosine kinases AXL, MET and VEGFR can mediate resistance to cetuximab. Cabozantinib, a multikinase inhibitor (MKI) targeting AXL/MET/VEGFR, has demonstrated antitumor activity in preclinical models of HNSCC. This investigator- initiated phase I trial evaluated the safety and efficacy of cetuximab plus cabozantinib in patients with recurrent/metastatic (R/M) HNSCC.
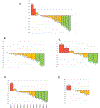
Materials and methods: Patients received cetuximab concurrently with cabozantinib daily on a 28-day cycle. Using a 3 + 3 dose-escalation design, the primary endpoint was to determine the maximally tolerated dose (MTD) of cabozantinib. Secondary endpoints included overall response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS) RESULTS: Among the 20 patients enrolled, most had prior disease progression on immune checkpoint inhibitors (95 %), platinum-based chemotherapy (95 %), and cetuximab (80 %). No dose-limiting toxicities were recorded and the MTD for cabozantinib was established to be 60 mg. Grade ≥ 3 adverse events occurred in 65 % of patients (n = 13). ORR was 20 %, with 4 partial responses (PRs). Two PRs were observed in cetuximab-naïve patients (n = 4), with an ORR of 50 % in this subgroup. In the overall population, DCR was 75 %, median PFS was 3.4 months and median OS was 8.1 months.
Conclusion: Cetuximab plus cabozantinib demonstrated a manageable toxicity profile and preliminary efficacy in patients with heavily treated R/M HNSCC. The combination of cetuximab with MKIs targeting the AXL/MET/VEGFR axis warrants further investigation, including in cetuximab-naïve patients.
Keywords: Cabozantinib; Cetuximab; Head and neck squamous cell cancer; Human papillomavirus-associated oropharyngeal squamous cell cancer; Immunotherapy resistance; Platinum resistance.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. Dec 15 2002;62(24):7350–6. - PubMed
-
- Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. Jun 1 2007;25(16):2171–7. doi: 10.1200/jco.2006.06.7447 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

