Merkel-cell carcinoma: ESMO-EURACAN Clinical Practice Guideline for diagnosis, treatment and follow-up
- PMID: 38796285
- PMCID: PMC11145756
- DOI: 10.1016/j.esmoop.2024.102977
Merkel-cell carcinoma: ESMO-EURACAN Clinical Practice Guideline for diagnosis, treatment and follow-up
Abstract
- •
This ESMO Clinical Practice Guideline provides key recommendations for managing Merkel-cell carcinoma (MCC).
- •
Recommendations are based on available scientific data and the multidisciplinary group of experts’ collective opinion.
- •
The guideline covers clinical and pathological diagnosis, staging and risk assessment, treatment and follow-up.
- •
Algorithms for the management of locoregional and inoperable/metastatic disease are provided.
- •
A multidisciplinary team with a high level of expertise in MCC should diagnose and make decisions about therapy.
Keywords: Merkel-cell carcinoma; clinical practice guideline; diagnosis; follow-up; treatment recommendation.
Conflict of interest statement
Disclosure IL reports personal fees for writing engagements for ESMO and Roche; personal and institutional fees as coordinating principal investigator (PI) for Agenus, Amgen, AstraZeneca, Bristol Myers Squibb (BMS), Celon, Incyte, Janssen, Menarini, MSD, Pfizer, Rhizen, Roche, RyVu and Siropa; institutional research grants from Agenus and Roche; non-financial interests as project lead for MSCI and board member for OECI; spouse has co-ownership of Clininote. JCB reports personal fees as a data safety monitoring board member for 4SC; personal fees as an advisory board member for Almirall, Amgen, Boehringer, InProTher, Merck, Recordati and Sanofi; institutional research grants from Alcedis, IQVIA and Merck; non-financial interests for receipt of product samples from 4SC. PAA reports personal fees for consultancy roles for 4SC, AstraZeneca, Bio-AI Health, BMS, Boehringer Ingelheim, Daiichi Sankyo, Idera, Immunocore, Italfarmaco, iTeos, Lunaphone, Medicenna, Merck Serono, MSD, Nektar, Nouscom, Novartis, Oncosec, Pfizer, Pfizer/Array, Pierre Fabre, Regeneron, Replimmune, Roche Genentech, Sandoz, Sanofi, Sun Pharma and ValoTx; personal fees for advisory roles for 4SC, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Eisai, Idera, Immunocore, iTeos, Merck Serono, MSD, Nektar, Novartis, Pfizer/Array, Pierre Fabre, Regeneron, Replimmune, Roche Genentech, Sandoz, Sanofi, Seagen, Sun Pharma and ValoTx; personal fees as an advisory board member from Erasca, iTeos, Replimmune and ValoTx; personal fees as travel support from Bio-AI-Health, Pfizer/Array and Replimmune; institutional funding from BMS, Pfizer/Array, Roche Genentech and Sanofi; non-financial interests as President of SCITO (Campania Society of ImmunoTherapy of Cancer) and Fondazione Melanoma Onlus Italy; non-financial interests as a member of the board of directors of the Society for Immunotherapy of Cancer (SITC; November 2017-December 2021) and a member of the steering committee for the Society for Melanoma Research (SMR); non-financial interests as a member of the Associazione Italiana di Oncologia Medica (AIOM), American Society of Clinical Oncology (ASCO), the European Organisation for Research and Treatment of Cancer (EORTC), Melanoma Cooperative Group, SITC and SMR. MV reports no potential conflicts of interests. ABl reports personal fees for advisory board membership from Merck (consultant for revision of approval of avelumab by French authorities). CL reports personal fees as an advisory board member from Amgen, BMS, Merck Serono, MSD, Novartis, Pierre Fabre, Roche and Sanofi; funding from BMS and Roche; non-financial interests for advisory roles for Amgen, BMS, Merck Serono, MSD, Novartis, Pierre Fabre, Roche and Sanofi; honoraria from Amgen, BMS, Incyte, MSD, Novartis, Pfizer, Pierre Fabre and Roche; travel/accommodation expenses from Avantis Medical Systems, BMS, Jazz Pharmaceuticals, MSD, Novartis, Pierre Fabre and Sanofi; research funding from BMS and Roche; participation on a data safety monitoring board/advisory board for InfalRx. EM reports no potential conflicts of interests. OH-V reports no potential conflicts of interests. MG reports personal fees for expert testimony from Almirall; personal fees as an advisory board member for GSK, Leo Pharma and Pfizer; personal fees as an invited speaker from GSK, Janssen, Lilly and Novartis; personal stocks/shares in BioNTech, Novo Nordisk and Siemens Healthineers; institutional fees as coordinating PI for Argenx and as local PI for Boehringer Ingelheim, Galderma, Janssen, Novartis and UCB Pharma; non-financial interests as Treasurer of the Deutsche Dermatologische Gesellschaft (DDG) and member of the board of directors of University Hospital Würzburg. HK reports no potential conflicts of interests. PN reports personal fees as an advisory board member for 4SC, BMS, IDEAYA Biosciences, Immunocore, Merck, Novartis and Pfizer; personal fees as an invited speaker for Novartis; institutional research support from Immunocore; non-financial interests as a steering committee member (personal) for 4SC and coordinating PI (institutional) for BMS, Immunocore, Ipsen, Merck, Novartis and Pfizer. PR reports personal fees as an invited speaker for AstraZeneca, BMS, Merck, MSD, Novartis, Pierre Fabre and Sanofi; personal fees as an advisory board member for Blueprint Medicines, BMS, Merck, MSD, Philogen, Pierre Fabre and Sanofi; institutional research grants from BMS and Pfizer; non-financial interests as President of the Polish Oncological Society and a member of the board of directors of the Polish Society of Surgical Oncology. MS reports personal fees as an invited speaker for Aristo-Pharma, Medac, Novartis and Takeda; personal fees as an advisory board member for Novartis and Takeda; personal fees as a PI for Amgen, Dermira, Eli Lilly and Company, Galderma, Parexel International and Regeneron; personal fees as a sub-investigator (SI) for OBWF NIO-PIB, Eli Lilly and Takeda. DS reports personal fees as an invited speaker for BMS, Merck Serono, MSD, Novartis, Roche and Sanofi; personal fees as an advisory board member for BMS, Immunocore, MSD, Neracare, Novartis, Pfizer, Philogen, Pierre Fabre and Sanofi/Regeneron; personal fees as a steering committee member for BMS, MSD and Novartis; institutional research grants from BMS and MSD; institutional fees as coordinating PI for BMS, MSD, Novartis and Pierre Fabre; institutional fees as local PI for Philogen and Sanofi; a non-financial interest as a member of the board of directors of EORTC-MG. JMP reports personal fees as an advisory board member for Astellas, AstraZeneca, BeiGene, BMS, Janssen, MSD, Roche and VCN Biosciences; personal and institutional research grants from BeiGene, BMS, Janssen, Mirati and Pfizer. FP reports no potential conflicts of interests. ACJvA reports institutional fees as an advisory board member for 4SC, Amgen, BMS, Merck–Pfizer, MSD–Merck, Novartis, Pierre Fabre, Provectus, Sanofi and Sirius Medical; institutional research grants from Amgen and Merck–Pfizer. ABe reports personal fees as an invited speaker for Amgen and HRA; personal fees as an advisory board member for Amgen, Astellas, Ipsen and Janssen; institutional funding from Astellas and Janssen; non-financial interests for receipt of product samples from Novartis and Sanofi.
Figures
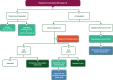

References
-
- Schadendorf D., Lebbe C., Zur Hausen A., et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53–69. - PubMed
-
- Coggshall K., Tello T.L., North J.P., et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78(3):433–442. - PubMed
-
- Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105(1):107–110. - PubMed

