Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer patients with brain metastases from the randomized DESTINY-Breast03 trial
- PMID: 38796287
- PMCID: PMC11145752
- DOI: 10.1016/j.esmoop.2024.102924
Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer patients with brain metastases from the randomized DESTINY-Breast03 trial
Abstract
Background: DESTINY-Breast03 is a randomized, multicenter, open-label, phase III study of trastuzumab deruxtecan (T-DXd) versus trastuzumab emtansine (T-DM1) in patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (mBC) previously treated with trastuzumab and a taxane. A statistically significant improvement in progression-free survival (PFS) versus T-DM1 was reported in the primary analysis. Here, we report exploratory efficacy data in patients with and without brain metastases (BMs) at baseline.
Patients and methods: Patients were randomly assigned 1 : 1 to receive T-DXd 5.4 mg/kg or T-DM1 3.6 mg/kg. Patients with clinically inactive/asymptomatic BMs were eligible. Lesions were measured as per modified RECIST, version 1.1. Outcomes included PFS by blinded independent central review (BICR), objective response rate (ORR), and intracranial ORR as per BICR.
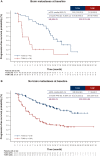
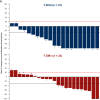
Results: As of 21 May 2021, 43/261 patients randomized to T-DXd and 39/263 patients randomized to T-DM1 had BMs at baseline, as per investigator assessment. Among patients with baseline BMs, 20/43 in the T-DXd arm and 19/39 in the T-DM1 arm had not received prior local BM treatment. For patients with BMs, median PFS was 15.0 months [95% confidence interval (CI) 12.5-22.2 months] for T-DXd versus 3.0 months (95% CI 2.8-5.8 months) for T-DM1; hazard ratio (HR) 0.25 (95% CI 0.13-0.45). For patients without BMs, median PFS was not reached (95% CI 22.4 months-not estimable) for T-DXd versus 7.1 months (95% CI 5.6-9.7 months) for T-DM1; HR 0.30 (95% CI 0.22-0.40). Confirmed systemic ORR was 67.4% for T-DXd versus 20.5% for T-DM1 and 82.1% for T-DXd versus 36.6% for T-DM1 for patients with and without BMs, respectively. Intracranial ORR was 65.7% with T-DXd versus 34.3% with T-DM1.
Conclusions: Patients with HER2-positive mBC whose disease progressed after trastuzumab and a taxane achieved a substantial benefit from treatment with T-DXd compared with T-DM1, including those with baseline BMs.
Keywords: HER2-positive; antibody–drug conjugates; brain metastases; metastatic breast cancer.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure SAH reports contracted paid research from Ambrx, Amgen, AstraZeneca, Arvinas, Bayer, CytomX, Daiichi Sankyo, Dignitana, Genentech/Roche, Gilead, GSK, Immunomedics, Eli Lilly, Macrogenics, Novartis, Pfizer, OBI Pharma, Orinove, Pieris, PUMA, Radius, Sanofi, Seagen, Zymeworks, and Phoenix Molecular Designs, Ltd; institutional grants from Ambrx and Samumed; research funding as national/international principal investigator from Novartis, Daiichi Sankyo, Seagen, and GNE/Roche; is a steering committee member of Novartis, Lilly, Daiichi Sankyo/AstraZeneca, GNE/Roche, and Sanofi; travel expenses paid for by Lilly (2019); and uncompensated consulting/advisory board memberships with 4DPharma, Ambrx, Amgen, Artios, Arvinas, Daiichi Sankyo, Dantari, Genentech/Roche, Immunomedics, Macrogenics, Eli Lilly, Novartis, NK Max, Pieris, Pyxis, Seagen, and Biotheranostics. SBK reports institutional research grants from Novartis, Sanofi Aventis, and Dongkook Pharmaceutical Company; consulting fees from Novartis, AstraZeneca, Lilly Dae Hwa Pharmaceutical Co, Ltd, IUS Abxis, and Daiichi Sankyo; participation on an advisory board with Lilly Dae Hwa Pharmaceutical Co, Ltd, IUS Abxis, Daiichi Sankyo, and Beigene; leadership or fiduciary role with ESMO Breast 2021-2023; and stocks or stock options from Genopeak and NeoGene TC. WPC reports payment or honoraria for lectures and presentations from Amgen, AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eli Lilly, Everest Medicine, Foundation Medicine Kyowa Kirin, Novartis, Pfizer, Roche, Sanofi, and Takeda; travel support from Amgen, AstraZeneca, Pfizer, and Roche; participation on data safety monitoring or advisory board with Amgen, AstraZeneca, Daiichi Sankyo, Everest Medicine, Eli Lilly, IMPACT Therapeutics, MSD, Novartis, Pfizer, Roche, and Sanofi. SAI reports grants or contracts from AstraZeneca, Boryung Pharm, Daewoong Pharmaceutical Co. Ltd, Eisai, Pfizer, and Roche; and consulting fees from AstraZeneca, Hanmi, Idience, Lilly, MSD, GSK, Pfizer, Novartis, Roche, and Daiichi Sankyo. YHP reports grants or contracts from AstraZeneca, Roche, Pfizer, Gencurix, and Novartis; consulting fees from Pfizer, AstraZeneca, Roche, MSD, Eisai, Novartis, Daiichi Sankyo, and Lilly; payment or honoraria from Pfizer, Roche, MSD, and Novartis; participation on data safety monitoring or advisory board with Roche, AstraZeneca, Eisai, MSD, Daiichi Sankyo, and Novartis. VP reports payment or honoraria from Daiichi Sankyo, Novartis, Pfizer, and AstraZeneca; travel support from Pfizer, Mundipharma, Daiichi Sankyo, and Lilly; and participation on an advisory board with Daiichi Sankyo. CFC reports participation on a data safety monitoring or advisory board with Roche, Pfizer, and Daiichi Sankyo. HI reports consulting fees from Daichi Sankyo, Chugai, AstraZeneca, Sanofi, Lilly, MSD, Pfizer, and Novartis; and payment or honoraria from Daiichi Sankyo, Chugai, AstraZeneca, Lilly, MSD, and Pfizer. EH reports institutional research and consulting fees for the present manuscript from Daiichi Sankyo and AstraZeneca; other institutional research grants from AbbVie, Acerta Pharma, ADC Therapeutics, AKESOBIO Australia, Amgen, Aravive, ArQule, Arvinas AtlasMedX, Black Diamond, Boehringer Ingelheim, Clovis, Compugen, Curis, CytomX, Dana Farber Cancer Institute, Deciphera, eFFECTOR Therapeutics, Ellipses Pharma, EMD Serono, Fochon, FujiFilm, G1 Therapeutics, H3 Biomedicine, Harpoon, Hutchinson MediPharma, Immunogen, Immunomedics, Incyte, InvestisBio, Jacobio, Karyopharm, Leap Therapeutics, Lilly, Lycera, Mabspace Biosciences, Macrogenics, MedImmune, Merck, Mersana, Merus, Millenium, Molecular Templates, Myriad Genetic Labs, Novartis, Nucana, Olema, OncoMed, Onconova Therapeutics, ORIC Pharmaceuticals, Orinove, Pfizer, PharmaMar, Pieris Pharmaceuticals, Pionyr Immunotherapeutics, Plexxikon, Radius Health, Regeneron, Repertoire Immune Medicine, Rgenix, Roche/Genentech, Seagen, Sermonix Pharmaceuticals, Shattuck Labs, Silverback, StemCentRx, Sutro, Syndax, Syros, Taiho, TapImmune, Tesaro, Treadwell Therapeutics, Verastem, Vincerx Pharma, Zenith Epigenetics, and Zymeworks; and consulting fees from Arcus, Arvinas, Black Diamond, Boehringer Ingelheim, CytomX, Dantari, Deciphera Pharmaceuticals, Eisai, H3 Biomedicine, iTeos, Janssen, Lilly, Loxo, Merck, Mersana, Novartis, Pfizer, Puma Biotechnology, Relay Therapeutics, Roche/Genentech, Seagen, and Silverback Therapeutics. GC reports support for the current manuscript from AstraZeneca and Daichii Sankyo; grants or contracts from Merck; consulting fees from Bristol Myers Squibb, Roche, Pfizer, Novartis, Lilly, AstraZeneca, Daichii Sankyo, Merck, Seagen, and Ellipsis; payments or honoraria from Pfizer and Lilly; and travel support from Roche and Lilly. BX reports consulting fees from AstraZeneca and Novartis and payments or honoraria for lectures from AstraZeneca, Pfizer, and Roche. AE reports stocks/stock options from and is an employee of Daiichi Sankyo. YL reports stocks/stock options from and was an employee of Daiichi Sankyo at the time of the study. JC is an employee of Daiichi Sankyo. EB was an employee of Daiichi Sankyo at the time of the study. KT is an employee of Daiichi Sankyo. SV reports stocks/stock options from and is an employee of AstraZeneca. JC reports support for the current work from Daiichi Sankyo and AstraZeneca; consulting fees from AstraZeneca, Athenex, Bioasis, BioInvent, Boehringer Ingelheim, Celgene, Cellestia, Clovis Oncology, Daiichi Sankyo, Ellipses, Erytech, GEMoaB, Gilead, GSK, Hibercell, Leuko, Lilly, Menarini, Merck Sharp & Dohme, Polyphor, Roche, Seagen, Zymeworks; payment or honoraria from Celegene, Daiichi Sankyo, Eisai, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Samsung Bioepis; travel support from AstraZeneca, Daiichi Sankyo, Eisai, Novartis, Pfizer, and Roche; stocks/stock options from Leuko (relative), MedSIR, and Nektar Pharmaceuticals. All other authors have declared no conflicts of interest.
Figures


References
-
- Harbeck N., Penault-Llorca F., Cortes J., et al. Breast cancer. Nat Rev Dis Primers. 2019;5(1):66. - PubMed
-
- Hurvitz S.A., O'Shaughnessy J., Mason G., et al. Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from SystHERs. Clin Cancer Res. 2019;25(8):2433–2441. - PubMed
-
- Brufsky A.M., Mayer M., Rugo H.S., et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–4843. - PubMed
-
- Ramakrishna N., Anders C.K., Lin N.U., et al. Management of advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol. 2022;40:2636–2655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

