Sustained antigen delivery improves germinal center reaction and increases antibody responses in neonatal mice
- PMID: 38796539
- PMCID: PMC11128021
- DOI: 10.1038/s41541-024-00875-3
Sustained antigen delivery improves germinal center reaction and increases antibody responses in neonatal mice
Abstract
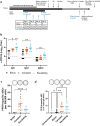
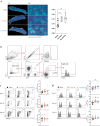
Neonates and young infants are known to have limited responses to pediatric vaccines due to reduced germinal center formation. Extended vaccine antigen dosing was previously shown to expand germinal center formation and improve humoral responses in adult mice. We report that sustained antigen delivery through sequential dosing overcomes neonatal limitations to form germinal center reactions and improves humoral immunity. Thus, vaccine strategies that extend the release of vaccine antigens may reduce the number of doses, and time needed, to achieve protective immunity in neonates and young infants.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources

