Population Pharmacokinetics of Bepirovirsen in Healthy Participants and Participants with Chronic Hepatitis B Virus Infection: Results from Phase 1, 2a, and 2b Studies
- PMID: 38796564
- PMCID: PMC11219612
- DOI: 10.1007/s40121-024-00980-9
Population Pharmacokinetics of Bepirovirsen in Healthy Participants and Participants with Chronic Hepatitis B Virus Infection: Results from Phase 1, 2a, and 2b Studies
Erratum in
-
Correction to: Population Pharmacokinetics of Bepirovirsen in Healthy Participants and Participants with Chronic Hepatitis B Virus Infection: Results from Phase 1, 2a, and 2b Studies.Infect Dis Ther. 2024 Dec;13(12):2663. doi: 10.1007/s40121-024-01066-2. Infect Dis Ther. 2024. PMID: 39460893 Free PMC article. No abstract available.
Abstract
Introduction: Bepirovirsen is a novel antisense oligonucleotide in development for chronic hepatitis B virus (HBV) infection therapy. Understanding the impact that clinical characteristics may have on bepirovirsen exposure is important for determining efficacious and well-tolerated dosing regimens. This analysis evaluated demographics and clinical characteristics associated with bepirovirsen exposure using a population pharmacokinetic (PK) analysis.
Methods: Population PK analyses were conducted using pooled data from three phase 1/2 clinical studies (NCT03020745/NCT02981602/NCT04449029) to construct a structural PK model for bepirovirsen that adequately described plasma concentration-time profiles and identify covariates that affect systemic exposure. The final population PK model was used to simulate bepirovirsen exposure measures to inform exposures at different dose levels and within different subpopulations.
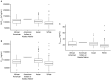
Results: Bepirovirsen PK data were well-described by a linear, three-compartment model with first-order absorption and absorption delay. Chronic HBV infection status, body weight, and Asian versus non-Asian race were key covariates included in the final model. Visual inspection of correlation scatter plots confirmed general agreement between observed and predicted data from the studies. In simulations, bepirovirsen systemic exposure was dosed proportionally and predicted to be almost completely washed out by 12 weeks following the final 300-mg dose. Differences in body weight, Asian race, or disease status did not result in clinically relevant differences in exposure.
Conclusions: This analysis demonstrated that the linear three-compartmental model accurately described bepirovirsen PK data. The lack of clinically relevant differences seen in exposure indicate that dose adjustments are not recommended for bepirovirsen based on demographics or clinical characteristics.
Keywords: Antisense oligonucleotide; Bepirovirsen; Chronic hepatitis B virus infection; Population pharmacokinetics.
© 2024. GSK Plc.
Conflict of interest statement
Ahmed Nader, Amir S Youssef, Kelong Han, and Mindy Magee are employees of GSK and hold stocks/shares in GSK. Mohamed Ismail is an employee of Enhanced Pharmacodynamics LLC and clinical pharmacology and pharmacometrics consultants supporting EOP2 analysis.
Figures


References
-
- World Health Organization. Hepatitis B factsheet 2023. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
-
- Ghany MG, Buti M, Lampertico P, Lee HM, AASLD-EASL HBV-HDV Treatment Endpoints Conference Faculty. Guidance on treatment endpoints and study design for clinical trials aiming to achieve cure in chronic hepatitis B and D: report from the 2022 AASLD-EASL HBV/HDV treatment endpoints conference. Hepatology. 2023. - PubMed
-
- Food and Drug Administration. Chronic hepatitis B virus infection: developing drugs for treatment guidance for industry 2022. https://www.fda.gov/media/117977/download.
Grants and funding
LinkOut - more resources
Full Text Sources

