Lateral parabrachial FoxP2 neurons regulate respiratory responses to hypercapnia
- PMID: 38796568
- PMCID: PMC11128025
- DOI: 10.1038/s41467-024-48773-5
Lateral parabrachial FoxP2 neurons regulate respiratory responses to hypercapnia
Abstract
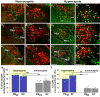
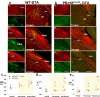
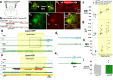
About half of the neurons in the parabrachial nucleus (PB) that are activated by CO2 are located in the external lateral (el) subnucleus, express calcitonin gene-related peptide (CGRP), and cause forebrain arousal. We report here, in male mice, that most of the remaining CO2-responsive neurons in the adjacent central lateral (PBcl) and Kölliker-Fuse (KF) PB subnuclei express the transcription factor FoxP2 and many of these neurons project to respiratory sites in the medulla. PBclFoxP2 neurons show increased intracellular calcium during wakefulness and REM sleep and in response to elevated CO2 during NREM sleep. Photo-activation of the PBclFoxP2 neurons increases respiration, whereas either photo-inhibition of PBclFoxP2 or genetic deletion of PB/KFFoxP2 neurons reduces the respiratory response to CO2 stimulation without preventing awakening. Thus, augmenting the PBcl/KFFoxP2 response to CO2 in patients with sleep apnea in combination with inhibition of the PBelCGRP neurons may avoid hypoventilation and minimize EEG arousals.
© 2024. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures




Update of
-
Lateral parabrachial FoxP2 neurons regulate respiratory responses to hypercapnia.Res Sq [Preprint]. 2023 May 5:rs.3.rs-2865756. doi: 10.21203/rs.3.rs-2865756/v1. Res Sq. 2023. Update in: Nat Commun. 2024 May 25;15(1):4475. doi: 10.1038/s41467-024-48773-5. PMID: 37205337 Free PMC article. Updated. Preprint.
References
-
- Edwards, B. A. et al. Increased upper airway resistance prolongs mechanical inspiratory time independent of changes in respiratory motor output during sleep in patients with obstructive sleep apnea. In American Thoracic Society 2012 International Conference A2435–A2435 (American Thoracic Society, California, 2012) 10.1164/ajrccm-conference.2012.185.1_meetingabstracts.a2435.
-
- Malhotra, A., Huang Y. & White, D. P. Upper airway mechanics: the influence of gender. In Proc. the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society (Engineering in Medicine and Biology) 1544–1545 (IEEE, 2002).
-
- Malhotra, A. Obstructive sleep apnea and central sleep apnea: epidemiology, pathophysiology, and risk factors. in ACCP Sleep Medicine Board Review: 4th Edition 193–200 (American College of Chest Physicians, 2009) 10.1378/smbr.4th.193.
MeSH terms
Substances
Grants and funding
- NS 072337/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 NS112175/NS/NINDS NIH HHS/United States
- NS112175/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- P01 HL149630/HL/NHLBI NIH HHS/United States
- P01 HL095491/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

