Impact of Ebola virus nucleoprotein on VP40 virus-like particle production: a computational approach
- PMID: 38796621
- PMCID: PMC11128010
- DOI: 10.1038/s42003-024-06300-8
Impact of Ebola virus nucleoprotein on VP40 virus-like particle production: a computational approach
Abstract
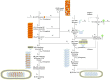
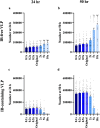
Ebola virus (EBOV) matrix protein VP40 can assemble and bud as virus-like particles (VLPs) when expressed alone in mammalian cells. Nucleoprotein (NP) could be recruited to VLPs as inclusion body (IB) when co-expressed, and increase VLP production. However, the mechanism behind it remains unclear. Here, we use a computational approach to study NP-VP40 interactions. Our simulations indicate that NP may enhance VLP production through stabilizing VP40 filaments and accelerating the VLP budding step. Further, both the relative timing and amount of NP expression compared to VP40 are important for the effective production of IB-containing VLPs. We predict that relative NP/VP40 expression ratio and time are important for efficient production of IB-containing VLPs. We conclude that disrupting the expression timing and amount of NP and VP40 could provide new avenues to treat EBOV infection. This work provides quantitative insights into EBOV proteins interactions and how virion generation and drug efficacy could be influenced.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Development of a Novel, Highly Sensitive System for Evaluating Ebola Virus Particle Formation.Viruses. 2025 Jul 19;17(7):1016. doi: 10.3390/v17071016. Viruses. 2025. PMID: 40733632 Free PMC article.
-
Contribution of ebola virus glycoprotein, nucleoprotein, and VP24 to budding of VP40 virus-like particles.J Virol. 2004 Jul;78(14):7344-51. doi: 10.1128/JVI.78.14.7344-7351.2004. J Virol. 2004. PMID: 15220407 Free PMC article.
-
Ubiquitin Ligase WWP1 Interacts with Ebola Virus VP40 To Regulate Egress.J Virol. 2017 Sep 27;91(20):e00812-17. doi: 10.1128/JVI.00812-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768865 Free PMC article.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff.Cochrane Database Syst Rev. 2016 Apr 19;4:CD011621. doi: 10.1002/14651858.CD011621.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2019 Jul 01;7:CD011621. doi: 10.1002/14651858.CD011621.pub3. PMID: 27093058 Free PMC article. Updated.
References
-
- Ebola Virus Disease Distribution Map: Cases of Ebola Virus Disease in Africa Since 1976 | History | Ebola (Ebola Virus Disease) | CDC. https://www.cdc.gov/vhf/ebola/history/distribution-map.html (2021).
-
- Aschenbrenner DS. Monoclonal antibody approved to treat Ebola. Am. J. Nurs. 2021;121:22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

