Photoinhibition of the hERG potassium channel PAS domain by ultraviolet light speeds channel closing
- PMID: 38796698
- PMCID: PMC11365103
- DOI: 10.1016/j.bpj.2024.05.024
Photoinhibition of the hERG potassium channel PAS domain by ultraviolet light speeds channel closing
Abstract
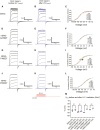
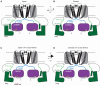
hERG potassium channels are critical for cardiac excitability. hERG channels have a Per-Arnt-Sim (PAS) domain at their N-terminus, and here, we examined the mechanism for PAS domain regulation of channel opening and closing (gating). We used TAG codon suppression to incorporate the noncanonical amino acid 4-benzoyl-L-phenylalanine (BZF), which is capable of forming covalent cross-links after photoactivation by ultraviolet (UV) light, at three locations (G47, F48, and E50) in the PAS domain. We found that hERG-G47BZF channels had faster closing (deactivation) when irradiated in the open state (at 0 mV) but showed no measurable changes when irradiated in the closed state (at -100 mV). hERG-F48BZF channels had slower activation, faster deactivation, and a marked rightward shift in the voltage dependence of activation when irradiated in the open (at 0 mV) or closed (at -100 mV) state. hERG-E50BZF channels had no measurable changes when irradiated in the open state (at 0 mV) but had slower activation, faster deactivation, and a rightward shift in the voltage dependence of activation when irradiated in the closed state (at -100mV), indicating that hERG-E50BZF had a state-dependent difference in UV photoactivation, which we interpret to mean that PAS underwent molecular motions between the open and closed states. Moreover, we propose that UV-dependent biophysical changes in hERG-G47BZF, F48BZF, and E50BZF were the direct result of photochemical cross-linking that reduced dynamic motions in the PAS domain and broadly stabilized the closed state relative to the open state of the channel.
Copyright © 2024 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Comment in
-
Locking hERG channels into place: Using photoreactive unnatural amino acids to study voltage gating.Biophys J. 2024 Aug 20;123(16):2358-2359. doi: 10.1016/j.bpj.2024.07.021. Epub 2024 Jul 20. Biophys J. 2024. PMID: 39033327 Free PMC article. No abstract available.
Similar articles
-
Photo-crosslinking hERG channels causes a U.V.-driven, state-dependent disruption of kinetics and voltage dependence of activation.bioRxiv [Preprint]. 2024 Jan 9:2024.01.09.574834. doi: 10.1101/2024.01.09.574834. bioRxiv. 2024. PMID: 38260338 Free PMC article. Preprint.
-
An intracellular hydrophobic nexus critical for hERG1 channel slow deactivation.Biophys J. 2024 Jul 16;123(14):2024-2037. doi: 10.1016/j.bpj.2024.01.010. Epub 2024 Jan 12. Biophys J. 2024. PMID: 38219015 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
Cited by
-
Unnatural Amino Acid Photo-Crosslinking Sheds Light on Gating of the Mechanosensitive Ion Channel OSCA1.2.Int J Mol Sci. 2025 Jul 23;26(15):7121. doi: 10.3390/ijms26157121. Int J Mol Sci. 2025. PMID: 40806253 Free PMC article.
-
Locking hERG channels into place: Using photoreactive unnatural amino acids to study voltage gating.Biophys J. 2024 Aug 20;123(16):2358-2359. doi: 10.1016/j.bpj.2024.07.021. Epub 2024 Jul 20. Biophys J. 2024. PMID: 39033327 Free PMC article. No abstract available.
-
Genetic Code Expansion for Mechanistic Studies in Ion Channels: An (Un)natural Union of Chemistry and Biology.Chem Rev. 2024 Oct 23;124(20):11523-11543. doi: 10.1021/acs.chemrev.4c00306. Epub 2024 Aug 29. Chem Rev. 2024. PMID: 39207057 Free PMC article. Review.
References
-
- Codding S.J., Trudeau M.C. In: Vol 2. Textbook of Ion Channels. Zheng J., Trudeau M.C., editors. Taylor and Francis/CRC Press; London: 2023. hERG Potassium Channels.
-
- Warmke J.W., Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc. Natl. Acad. Sci. USA. 1994;91:3438–3442. http://www.ncbi.nlm.nih.gov/pubmed/8159766 - PMC - PubMed
-
- Guy H.R., Durell S.R., et al. Ganetzky B. Similarities in amino acid sequences of Drosophila eag and cyclic nucleotide-gated channels. Science. 1991;254:730. http://www.ncbi.nlm.nih.gov/pubmed/1658932 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

