In vivo adenine base editing rescues adrenoleukodystrophy in a humanized mouse model
- PMID: 38796705
- PMCID: PMC11286820
- DOI: 10.1016/j.ymthe.2024.05.027
In vivo adenine base editing rescues adrenoleukodystrophy in a humanized mouse model
Abstract
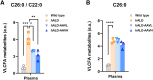
X-linked adrenoleukodystrophy (ALD), an inherited neurometabolic disorder caused by mutations in ABCD1, which encodes the peroxisomal ABC transporter, mainly affects the brain, spinal cord, adrenal glands, and testes. In ALD patients, very-long-chain fatty acids (VLCFAs) fail to enter the peroxisome and undergo subsequent β-oxidation, resulting in their accumulation in the body. It has not been tested whether in vivo base editing or prime editing can be harnessed to ameliorate ALD. We developed a humanized mouse model of ALD by inserting a human cDNA containing the pathogenic variant into the mouse Abcd1 locus. The humanized ALD model showed increased levels of VLCFAs. To correct the mutation, we tested both base editing and prime editing and found that base editing using ABE8e(V106W) could correct the mutation in patient-derived fibroblasts at an efficiency of 7.4%. Adeno-associated virus (AAV)-mediated systemic delivery of NG-ABE8e(V106W) enabled robust correction of the pathogenic variant in the mouse brain (correction efficiency: ∼5.5%), spinal cord (∼5.1%), and adrenal gland (∼2%), leading to a significant reduction in the plasma levels of C26:0/C22:0. This established humanized mouse model and the successful correction of the pathogenic variant using a base editor serve as a significant step toward treating human ALD disease.
Keywords: ABCD1; CRISPR; adenine base editing; adrenoleukodystrophy; genome editing; humanized mouse model; very-long-chain fatty acid.
Copyright © 2024 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Wiesinger C., Kunze M., Regelsberger G., Forss-Petter S., Berger J. Impaired very long-chain acyl-CoA beta-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dysfunction. J. Biol. Chem. 2013;288:19269–19279. doi: 10.1074/jbc.M112.445445. - DOI - PMC - PubMed
-
- Kohler W., Engelen M., Eichler F., Lachmann R., Fatemi A., Sampson J., Salsano E., Gamez J., Molnar M.J., Pascual S., et al. Safety and efficacy of leriglitazone for preventing disease progression in men with adrenomyeloneuropathy (ADVANCE): a randomised, double-blind, multi-centre, placebo-controlled phase 2-3 trial. Lancet Neurol. 2023;22:127–136. doi: 10.1016/S1474-4422(22)00495-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

