Evolution of biotechnological advances and regenerative therapies for endometrial disorders: a systematic review
- PMID: 38796750
- PMCID: PMC11369227
- DOI: 10.1093/humupd/dmae013
Evolution of biotechnological advances and regenerative therapies for endometrial disorders: a systematic review
Abstract
Background: The establishment and maintenance of pregnancy depend on endometrial competence. Asherman syndrome (AS) and intrauterine adhesions (IUA), or endometrial atrophy (EA) and thin endometrium (TE), can either originate autonomously or arise as a result from conditions (i.e. endometritis or congenital hypoplasia), or medical interventions (e.g. surgeries, hormonal therapies, uterine curettage or radiotherapy). Affected patients may present an altered or inadequate endometrial lining that hinders embryo implantation and increases the risk of poor pregnancy outcomes and miscarriage. In humans, AS/IUA and EA/TE are mainly treated with surgeries or pharmacotherapy, however the reported efficacy of these therapeutic approaches remains unclear. Thus, novel regenerative techniques utilizing stem cells, growth factors, or tissue engineering have emerged to improve reproductive outcomes.
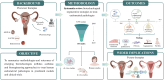
Objective and rationale: This review comprehensively summarizes the methodologies and outcomes of emerging biotechnologies (cellular, acellular, and bioengineering approaches) to treat human endometrial pathologies. Regenerative therapies derived from human tissues or blood which were studied in preclinical models (in vitro and in vivo) and clinical trials are discussed.
Search methods: A systematic search of full-text articles available in PubMed and Embase was conducted to identify original peer-reviewed studies published in English between January 2000 and September 2023. The search terms included: human, uterus, endometrium, Asherman syndrome, intrauterine adhesions, endometrial atrophy, thin endometrium, endometritis, congenital hypoplasia, curettage, radiotherapy, regenerative therapy, bioengineering, stem cells, vesicles, platelet-rich plasma, biomaterials, microfluidic, bioprinting, organoids, hydrogel, scaffold, sheet, miRNA, sildenafil, nitroglycerine, aspirin, growth hormone, progesterone, and estrogen. Preclinical and clinical studies on cellular, acellular, and bioengineering strategies to repair or regenerate the human endometrium were included. Additional studies were identified through manual searches.
Outcomes: From a total of 4366 records identified, 164 studies (3.8%) were included for systematic review. Due to heterogeneity in the study design and measured outcome parameters in both preclinical and clinical studies, the findings were evaluated qualitatively and quantitatively without meta-analysis. Groups using stem cell-based treatments for endometrial pathologies commonly employed mesenchymal stem cells (MSCs) derived from the human bone marrow or umbilical cord. Alternatively, acellular therapies based on platelet-rich plasma (PRP) or extracellular vesicles are gaining popularity. These are accompanied by the emergence of bioengineering strategies based on extracellular matrix (ECM)-derived hydrogels or synthetic biosimilars that sustain local delivery of cells and growth factors, reporting promising results. Combined therapies that target multiple aspects of tissue repair and regeneration remain in preclinical testing but have shown translational value. This review highlights the myriad of therapeutic material sources, administration methods, and carriers that have been tested.
Wider implications: Therapies that promote endometrial proliferation, vascular development, and tissue repair may help restore endometrial function and, ultimately, fertility. Based on the existing evidence, cost, accessibility, and availability of the therapies, we propose the development of triple-hit regenerative strategies, potentially combining high-yield MSCs (e.g. from bone marrow or umbilical cord) with acellular treatments (PRP), possibly integrated in ECM hydrogels. Advances in biotechnologies together with insights from preclinical models will pave the way for developing personalized treatment regimens for patients with infertility-causing endometrial disorders such as AS/IUA, EA/TE, and endometritis.
Registration number: https://osf.io/th8yf/.
Keywords: Asherman síndrome; acellular therapy; bioengineering; endometrial atrophy; endometritis; endometrium; fertility restoration; intrauterine adhesions; stem cell therapy; thin endometrium.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
E.S. is a grant recipient or holds a contract with the Foundation for Embryonic Competence.
Figures




References
-
- Acharya S, Yasmin E, Balen AH.. The use of a combination of pentoxifylline and tocopherol in women with a thin endometrium undergoing assisted conception therapies—a report of 20 cases. Hum Fertil (Camb) 2009;12:198–203. - PubMed
-
- Ahmed ME, Amer MI, Ahmed WE.. Platelet rich plasma following hysteroscopic adhesolysis: a randomized clinical trial. Int J Reprod Contracept Obstet Gynecol 2021;10:433–438.
-
- Amui J, Check JH, Cohen R.. Successful twin pregnancy in a donor oocyte recipient despite a maximum endometrial thickness in the late proliferative phase of 4 mm. Clin Exp Obstet Gynecol 2011;38:328–329. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

