Regulation of Hippo-YAP signaling axis by Isoalantolactone suppresses tumor progression in cholangiocarcinoma
- PMID: 38797019
- PMCID: PMC11152753
- DOI: 10.1016/j.tranon.2024.101971
Regulation of Hippo-YAP signaling axis by Isoalantolactone suppresses tumor progression in cholangiocarcinoma
Abstract
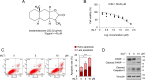
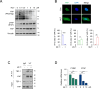
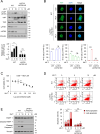
Cholangiocarcinoma (CCA) is a devastating malignancy characterized by aggressive tumor growth and limited treatment options. Dysregulation of the Hippo signaling pathway and its downstream effector, Yes-associated protein (YAP), has been implicated in CCA development and progression. In this study, we investigated the effects of Isoalantolactone (IALT) on CCA cells to elucidate its effect on YAP activity and its potential clinical significance. Our findings demonstrate that IALT exerts cytotoxic effects, induces apoptosis, and modulates YAP signaling in SNU478 cells. We further confirmed the involvement of the canonical Hippo pathway by generating LATS1/LATS2 knockout cells, highlighting the dependence of IALT-mediated apoptosis and YAP phosphorylation on the Hippo-LATS signaling axis. In addition, IALT suppressed cell growth and migration, partially dependent on YAP-TEAD activity. These results provide insights into the therapeutic potential of targeting YAP in CCA and provide a rationale for developing of YAP-targeted therapies for this challenging malignancy.
Keywords: Apoptosis; Cell growth; Cell migration; Cholangiocarcinoma; Hippo-YAP pathway; Isoalantolactone.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare no competing interests.
Figures

References
-
- Banales J.M., Marin J.J.G., Lamarca A., Rodrigues P.M., Khan S.A., Roberts L.R., Cardinale V., Carpino G., Andersen J.B., Braconi C., Calvisi D.F., Perugorria M.J., Fabris L., Boulter L., Macias R.I.R., Gaudio E., Alvaro D., Gradilone S.A., Strazzabosco M., Marzioni M., Coulouarn C., Fouassier L., Raggi C., Invernizzi P., Mertens J.C., Moncsek A., Rizvi S., Heimbach J., Koerkamp B.G., Bruix J., Forner A., Bridgewater J., Valle J.W., Gores G.J. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020;17:557–588. - PMC - PubMed
-
- Bum-Erdene K., Zhou D., Gonzalez-Gutierrez G., Ghozayel M.K., Si Y., Xu D., Shannon H.E., Bailey B.J., Corson T.W., Pollok K.E., Wells C.D., Meroueh S.O. Small-molecule covalent modification of conserved cysteine leads to allosteric inhibition of the TEAD·Yap protein-protein interaction. Cell Chem. Biol. 2019;26:378–389. e313. - PubMed
LinkOut - more resources
Full Text Sources

