Hepatocellular RECK as a Critical Regulator of Metabolic Dysfunction-associated Steatohepatitis Development
- PMID: 38797477
- PMCID: PMC11278626
- DOI: 10.1016/j.jcmgh.2024.101365
Hepatocellular RECK as a Critical Regulator of Metabolic Dysfunction-associated Steatohepatitis Development
Abstract
Background & aims: Reversion-inducing cysteine-rich protein with Kazal motifs (RECK) is an extracellular matrix regulator with anti-fibrotic effects. However, its expression and role in metabolic dysfunction-associated steatohepatitis (MASH) and hepatic fibrosis are poorly understood.
Methods: We generated a novel transgenic mouse model with RECK overexpression specifically in hepatocytes to investigate its role in Western diet (WD)-induced liver disease. Proteomic analysis and in vitro studies were performed to mechanistically link RECK to hepatic inflammation and fibrosis.
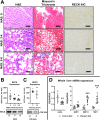
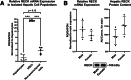
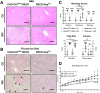
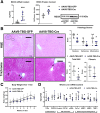
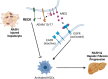
Results: Our results show that RECK expression is significantly decreased in liver biopsies from human patients diagnosed with MASH and correlated negatively with severity of metabolic dysfunction-associated steatotic liver disease (MASLD) and fibrosis. Similarly, RECK expression is downregulated in WD-induced MASH in wild-type mice. Hepatocyte-specific RECK overexpression significantly reduced hepatic pathology in WD-induced liver injury. Proteomic analysis highlighted changes in extracellular matrix and cell-signaling proteins. In vitro mechanistic studies linked RECK induction to reduced ADAM10 (a disintegrin and metalloproteinase domain-containing protein 10) and ADAM17 activity, amphiregulin release, epidermal growth factor receptor activation, and stellate cell activation.
Conclusion: Our in vivo and mechanistic in vitro studies reveal that RECK is a novel upstream regulator of inflammation and fibrosis in the diseased liver, its induction is hepatoprotective, and thus highlights its potential as a novel therapeutic in MASH.
Keywords: Amphiregulin; Epidermal Growth Factor Receptor; Extracellular Matrix; Fatty Liver Disease; MASH; MASLD; NAFLD; NASH; Reversion-inducing Cysteine-rich Protein With Kazal Motifs; Steatosis.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Figures









References
-
- Eslam M., Sanyal A.J., George J., International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. - PubMed
-
- Zou B., Yeo Y.H., Nguyen V.H., Cheung R., Ingelsson E., Nguyen M.H. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999-2016. J Intern Med. 2020;288:139–151. - PubMed
-
- Nagini S. RECKing MMP: relevance of reversion-inducing cysteine-rich protein with kazal motifs as a prognostic marker and therapeutic target for cancer (a review) Anticancer Agents Med Chem. 2012;12:718–725. - PubMed
-
- Somanna N.K., Valente A.J., Krenz M., et al. Histone deacetyltransferase inhibitors Trichostatin A and Mocetinostat differentially regulate MMP9, IL-18 and RECK expression, and attenuate Angiotensin II-induced cardiac fibroblast migration and proliferation. Hypertens Res. 2016;39:709–716. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

