Localization of stuttering based on causal brain lesions
- PMID: 38797521
- PMCID: PMC11146419
- DOI: 10.1093/brain/awae059
Localization of stuttering based on causal brain lesions
Abstract
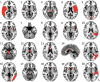
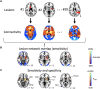
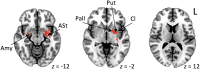
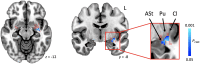
Stuttering affects approximately 1 in 100 adults and can result in significant communication problems and social anxiety. It most often occurs as a developmental disorder but can also be caused by focal brain damage. These latter cases may lend unique insight into the brain regions causing stuttering. Here, we investigated the neuroanatomical substrate of stuttering using three independent datasets: (i) case reports from the published literature of acquired neurogenic stuttering following stroke (n = 20, 14 males/six females, 16-77 years); (ii) a clinical single study cohort with acquired neurogenic stuttering following stroke (n = 20, 13 males/seven females, 45-87 years); and (iii) adults with persistent developmental stuttering (n = 20, 14 males/six females, 18-43 years). We used the first two datasets and lesion network mapping to test whether lesions causing acquired stuttering map to a common brain network. We then used the third dataset to test whether this lesion-based network was relevant to developmental stuttering. In our literature dataset, we found that lesions causing stuttering occurred in multiple heterogeneous brain regions, but these lesion locations were all functionally connected to a common network centred around the left putamen, including the claustrum, amygdalostriatal transition area and other adjacent areas. This finding was shown to be specific for stuttering (PFWE < 0.05) and reproducible in our independent clinical cohort of patients with stroke-induced stuttering (PFWE < 0.05), resulting in a common acquired stuttering network across both stroke datasets. Within the common acquired stuttering network, we found a significant association between grey matter volume and stuttering impact for adults with persistent developmental stuttering in the left posteroventral putamen, extending into the adjacent claustrum and amygdalostriatal transition area (PFWE < 0.05). We conclude that lesions causing acquired neurogenic stuttering map to a common brain network, centred to the left putamen, claustrum and amygdalostriatal transition area. The association of this lesion-based network with symptom severity in developmental stuttering suggests a shared neuroanatomy across aetiologies.
Keywords: acquired neurogenic stuttering; amygdala; claustrum; lesion network mapping; persistent developmental stuttering; putamen.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
M.D.F. has intellectual property on the use of brain connectivity imaging to analyse lesions and guide brain stimulation and is a consultant for Magnus Medical, Soterix, Abbott, and Boston Scientific. J.J. has received conference travel support from Abbvie, Abbott and Insightec, lecturer honoraria from Lundbeck and Novartis, and consultation fees from Summaryx and Adamant Health.
Figures

References
-
- Craig A, Tran Y. Trait and social anxiety in adults with chronic stuttering: Conclusions following meta-analysis. J Fluency Disord. 2014;40:35–43. - PubMed
-
- Iverach L, Rapee RM, Wong QJ, Lowe R. Maintenance of social anxiety in stuttering: A cognitive-behavioral model. Am J Speech Lang Pathol. 2017;26:540–556. - PubMed
-
- Matrone G. Moving past my stutter. Science. 2022;378:106. - PubMed
MeSH terms
Grants and funding
- University of Turku
- Finnish Parkinson Foundation
- M1180/Royal Society of New Zealand Marsden Fund
- Ellison/Baszucki Family Foundation
- R01 MH113929/MH/NIMH NIH HHS/United States
- R21 NS123813/NS/NINDS NIH HHS/United States
- K23MH120510/MH/NIMH NIH HHS/United States
- R01 DC007683/DC/NIDCD NIH HHS/United States
- R01MH113929/Simons Foundation Autism Research Initiative
- R21 MH126271/MH/NIMH NIH HHS/United States
- Instrumentarium Research Foundation
- R56 AG069086/AG/NIA NIH HHS/United States
- Sigrid Juselius Foundation
- K23 MH120510/MH/NIMH NIH HHS/United States
- R01 NS127892/NS/NINDS NIH HHS/United States
- Finnish Medical Foundation
- Turku University Hospital
- Kaye Family Research Endowment
- R01 DC007683/NH/NIH HHS/United States
- Health Research Council of New Zealand Charles Hercus Career Development Fellowship
- Manley Family
- Natural Sciences and Engineering Research Council
LinkOut - more resources
Full Text Sources
Medical

