The hepatoprotective effect of 4-phenyltetrahydroquinolines on carbon tetrachloride induced hepatotoxicity in rats through autophagy inhibition
- PMID: 38797855
- PMCID: PMC11129499
- DOI: 10.1186/s40659-024-00510-4
The hepatoprotective effect of 4-phenyltetrahydroquinolines on carbon tetrachloride induced hepatotoxicity in rats through autophagy inhibition
Abstract
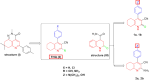
Background: The liver serves as a metabolic hub within the human body, playing a crucial role in various essential functions, such as detoxification, nutrient metabolism, and hormone regulation. Therefore, protecting the liver against endogenous and exogenous insults has become a primary focus in medical research. Consequently, the potential hepatoprotective properties of multiple 4-phenyltetrahydroquinolines inspired us to thoroughly study the influence of four specially designed and synthesized derivatives on carbon tetrachloride (CCl4)-induced liver injury in rats.
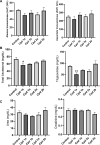
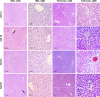
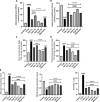
Methods and results: Seventy-seven Wistar albino male rats weighing 140 ± 18 g were divided into eleven groups to investigate both the toxicity profile and the hepatoprotective potential of 4-phenyltetrahydroquinolines. An in-vivo hepatotoxicity model was conducted using CCl4 (1 ml/kg body weight, a 1:1 v/v mixture with corn oil, i.p.) every 72 h for 14 days. The concurrent treatment of rats with our newly synthesized compounds (each at a dose of 25 mg/kg body weight, suspended in 0.5% CMC, p.o.) every 24 h effectively lowered transaminases, preserved liver tissue integrity, and mitigated oxidative stress and inflammation. Moreover, the histopathological examination of liver tissues revealed a significant reduction in liver fibrosis, which was further supported by the immunohistochemical analysis of α-SMA. Additionally, the expression of the apoptotic genes BAX and BCL2 was monitored using real-time PCR, which showed a significant decrease in liver apoptosis. Further investigations unveiled the ability of the compounds to significantly decrease the expression of autophagy-related proteins, Beclin-1 and LC3B, consequently inhibiting autophagy. Finally, our computer-assisted simulation dockingonfirmed the obtained experimental activities.
Conclusion: Our findings suggest that derivatives of 4-phenyltetrahydroquinoline demonstrate hepatoprotective properties in CCl4-induced liver damage and fibrosis in rats. The potential mechanism of action may be due to the inhibition of autophagy in liver cells.
Keywords: 4-phenyltetrahydroquinoline; Autophagy; CCL4-induced hepatotoxicity; CYP2E1; HepG2; Hepatoprotective; Liver injury; Tacrine derivatives.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Hepatoprotective efficacy of Lagenaria siceraria seeds oil against experimentally carbon tetrachloride-induced toxicity.Open Vet J. 2024 Aug;14(8):2016-2028. doi: 10.5455/OVJ.2024.v14.i8.31. Epub 2024 Aug 31. Open Vet J. 2024. PMID: 39308725 Free PMC article.
-
Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats.J Ethnopharmacol. 2013 Dec 12;150(3):973-81. doi: 10.1016/j.jep.2013.09.048. Epub 2013 Oct 17. J Ethnopharmacol. 2013. PMID: 24140589
-
Kadsura heteroclita stem ethanol extract protects against carbon tetrachloride-induced liver injury in mice via suppression of oxidative stress, inflammation, and apoptosis.J Ethnopharmacol. 2021 Mar 1;267:113496. doi: 10.1016/j.jep.2020.113496. Epub 2020 Oct 19. J Ethnopharmacol. 2021. PMID: 33091494
-
Putative abrogation impacts of Ajwa seeds on oxidative damage, liver dysfunction and associated complications in rats exposed to carbon tetrachloride.Mol Biol Rep. 2021 Jun;48(6):5305-5318. doi: 10.1007/s11033-021-06544-1. Epub 2021 Jul 9. Mol Biol Rep. 2021. PMID: 34244886
-
An Insight into Different Experimental Models used for Hepatoprotective Studies: A Review.Curr Drug Discov Technol. 2024;21(4):e191223224660. doi: 10.2174/0115701638278844231214115102. Curr Drug Discov Technol. 2024. PMID: 39206705 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

