Dynamic hydrogel-metal-organic framework system promotes bone regeneration in periodontitis through controlled drug delivery
- PMID: 38797862
- PMCID: PMC11129436
- DOI: 10.1186/s12951-024-02555-9
Dynamic hydrogel-metal-organic framework system promotes bone regeneration in periodontitis through controlled drug delivery
Abstract
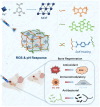
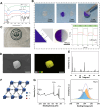
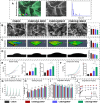
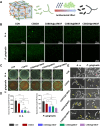
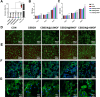
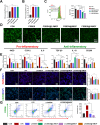
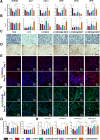
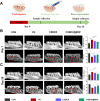
Periodontitis is a prevalent chronic inflammatory disease, which leads to gradual degradation of alveolar bone. The challenges persist in achieving effective alveolar bone repair due to the unique bacterial microenvironment's impact on immune responses. This study explores a novel approach utilizing Metal-Organic Frameworks (MOFs) (comprising magnesium and gallic acid) for promoting bone regeneration in periodontitis, which focuses on the physiological roles of magnesium ions in bone repair and gallic acid's antioxidant and immunomodulatory properties. However, the dynamic oral environment and irregular periodontal pockets pose challenges for sustained drug delivery. A smart responsive hydrogel system, integrating Carboxymethyl Chitosan (CMCS), Dextran (DEX) and 4-formylphenylboronic acid (4-FPBA) was designed to address this problem. The injectable self-healing hydrogel forms a dual-crosslinked network, incorporating the MOF and rendering its on-demand release sensitive to reactive oxygen species (ROS) levels and pH levels of periodontitis. We seek to analyze the hydrogel's synergistic effects with MOFs in antibacterial functions, immunomodulation and promotion of bone regeneration in periodontitis. In vivo and in vitro experiment validated the system's efficacy in inhibiting inflammation-related genes and proteins expression to foster periodontal bone regeneration. This dynamic hydrogel system with MOFs, shows promise as a potential therapeutic avenue for addressing the challenges in bone regeneration in periodontitis.
Keywords: Bone regeneration; Drug delivery; Hydrogel; Metal-organic frameworks; Periodontitis.
© 2024. The Author(s).
Conflict of interest statement
All authors disclosed no relevant relationships.
Figures
Similar articles
-
A Microenvironment-Responsive, Controlled Release Hydrogel Delivering Embelin to Promote Bone Repair of Periodontitis via Anti-Infection and Osteo-Immune Modulation.Adv Sci (Weinh). 2024 Sep;11(34):e2403786. doi: 10.1002/advs.202403786. Epub 2024 Jul 8. Adv Sci (Weinh). 2024. PMID: 38978324 Free PMC article.
-
ROS-responsive hydrogel for bone regeneration: Controlled dimethyl fumarate release to reduce inflammation and enhance osteogenesis.Acta Biomater. 2025 Mar 15;195:183-200. doi: 10.1016/j.actbio.2025.02.026. Epub 2025 Feb 14. Acta Biomater. 2025. PMID: 39956305
-
Chitosan-P407-PNIPAM hydrogel loaded with AgNPs/lipid complex for antibacterial, inflammation regulation and alveolar bone regeneration in periodontitis treatment.Int J Biol Macromol. 2025 May;307(Pt 4):142080. doi: 10.1016/j.ijbiomac.2025.142080. Epub 2025 Mar 17. Int J Biol Macromol. 2025. PMID: 40107529
-
Hydrogel design and applications for periodontitis therapy: A review.Int J Biol Macromol. 2025 Jan;284(Pt 1):137893. doi: 10.1016/j.ijbiomac.2024.137893. Epub 2024 Nov 19. Int J Biol Macromol. 2025. PMID: 39571840 Review.
-
[Latest Findings on Hydrogel Drug Delivery Systems in the Treatment of Periodontitis].Sichuan Da Xue Xue Bao Yi Xue Ban. 2023 Jul;54(4):721-725. doi: 10.12182/20230760203. Sichuan Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37545063 Free PMC article. Review. Chinese.
Cited by
-
Recent advances of chitosan-based composite hydrogel materials in application of bone tissue engineering.Heliyon. 2024 Sep 7;10(19):e37431. doi: 10.1016/j.heliyon.2024.e37431. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39381099 Free PMC article. Review.
-
Regulating periodontal disease with smart stimuli-responsive systems: Antimicrobial activity, immunomodulation, periodontium regeneration.Mater Today Bio. 2025 May 15;32:101863. doi: 10.1016/j.mtbio.2025.101863. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40496719 Free PMC article. Review.
-
Application of a novel thermal/pH-responsive antibacterial paeoniflorin hydrogel crosslinked with amino acids for accelerated diabetic foot ulcers healing.Mater Today Bio. 2025 Apr 5;32:101736. doi: 10.1016/j.mtbio.2025.101736. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40255581 Free PMC article.
-
Advances in Local Drug Delivery for Periodontal Treatment: Present Strategies and Future Directions.Biomolecules. 2025 Jun 19;15(6):903. doi: 10.3390/biom15060903. Biomolecules. 2025. PMID: 40563543 Free PMC article. Review.
-
Injectable hydrogels with ROS-triggered drug release enable the co-delivery of antibacterial agent and anti-inflammatory nanoparticle for periodontitis treatment.J Nanobiotechnology. 2025 Mar 12;23(1):205. doi: 10.1186/s12951-025-03275-4. J Nanobiotechnology. 2025. PMID: 40075491 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

