FLN-2 functions in parallel to linker of nucleoskeleton and cytoskeleton complexes and CDC-42/actin pathways during P-cell nuclear migration through constricted spaces in Caenorhabditis elegans
- PMID: 38797871
- PMCID: PMC11228842
- DOI: 10.1093/genetics/iyae071
FLN-2 functions in parallel to linker of nucleoskeleton and cytoskeleton complexes and CDC-42/actin pathways during P-cell nuclear migration through constricted spaces in Caenorhabditis elegans
Abstract
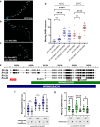
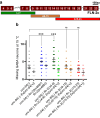
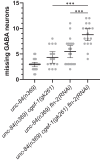
Nuclear migration through narrow constrictions is important for development, metastasis, and proinflammatory responses. Studies performed in tissue culture cells have implicated linker of nucleoskeleton and cytoskeleton (LINC) complexes, microtubule motors, the actin cytoskeleton, and nuclear envelope repair machinery as important mediators of nuclear movements through constricted spaces. However, little is understood about how these mechanisms operate to move nuclei in vivo. In Caenorhabditis elegans larvae, six pairs of hypodermal P cells migrate from lateral to ventral positions through a constricted space between the body wall muscles and the cuticle. P-cell nuclear migration is mediated in part by LINC complexes using a microtubule-based pathway and by an independent CDC-42/actin-based pathway. However, when both LINC complex and actin-based pathways are knocked out, many nuclei still migrate, suggesting the existence of additional pathways. Here, we show that FLN-2 functions in a third pathway to mediate P-cell nuclear migration. The predicted N-terminal actin-binding domain in FLN-2 that is found in canonical filamins is dispensable for FLN-2 function; this and structural predictions suggest that FLN-2 does not function as a filamin. The immunoglobulin-like repeats 4-8 of FLN-2 were necessary for P-cell nuclear migration. Furthermore, in the absence of the LINC complex component unc-84, fln-2 mutants had an increase in P-cell nuclear rupture. We conclude that FLN-2 functions to maintain the integrity of the nuclear envelope in parallel with the LINC complex and CDC-42/actin-based pathways to move P-cell nuclei through constricted spaces.
Keywords: Cdc42; LINC complexes; enhancers; filamin; hypodermal development; nuclear migration.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Genetics Society of America. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Conflicts of interest The author(s) declare no conflict of interest.
Figures




Update of
-
FLN-2 functions in parallel to LINC complexes and Cdc42/actin pathways during P-cell nuclear migration through constricted spaces in Caenorhabditis elegans.bioRxiv [Preprint]. 2023 Aug 6:2023.08.04.552041. doi: 10.1101/2023.08.04.552041. bioRxiv. 2023. Update in: Genetics. 2024 Jul 8;227(3):iyae071. doi: 10.1093/genetics/iyae071. PMID: 37577634 Free PMC article. Updated. Preprint.
References
-
- Altun ZF, Hall DH. 2024. Handbook of C. elegans Anatomy. In WormAtlas.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

