This is a preprint.
Rapid, biochemical tagging of cellular activity history in vivo
- PMID: 38798353
- PMCID: PMC11118534
- DOI: 10.1101/2023.09.06.556431
Rapid, biochemical tagging of cellular activity history in vivo
Update in
-
Rapid, biochemical tagging of cellular activity history in vivo.Nat Methods. 2024 Sep;21(9):1725-1735. doi: 10.1038/s41592-024-02375-7. Epub 2024 Aug 5. Nat Methods. 2024. PMID: 39103446 Free PMC article.
Abstract
Intracellular calcium (Ca2+) is ubiquitous to cell signaling across all biology. While existing fluorescent sensors and reporters can detect activated cells with elevated Ca2+ levels, these approaches require implants to deliver light to deep tissue, precluding their noninvasive use in freely-behaving animals. Here we engineered an enzyme-catalyzed approach that rapidly and biochemically tags cells with elevated Ca2+ in vivo. Ca2+-activated Split-TurboID (CaST) labels activated cells within 10 minutes with an exogenously-delivered biotin molecule. The enzymatic signal increases with Ca2+ concentration and biotin labeling time, demonstrating that CaST is a time-gated integrator of total Ca2+ activity. Furthermore, the CaST read-out can be performed immediately after activity labeling, in contrast to transcriptional reporters that require hours to produce signal. These capabilities allowed us to apply CaST to tag prefrontal cortex neurons activated by psilocybin, and to correlate the CaST signal with psilocybin-induced head-twitch responses in untethered mice.
Conflict of interest statement
COMPETING INTERESTS STATEMENT D.E.O. is a co-founder of Delix Therapeutics, Inc. and serves as the chief innovation officer and head of the scientific advisory board. All other authors declare no other competing interests.
Figures

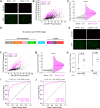
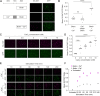

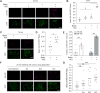

References
-
- Grynkiewicz G., Poenie M. & Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of biological chemistry 260, 3440–3450 (1985). - PubMed
-
- Inoue M. et al. Rational design of a high-affinity, fast, red calcium indicator R-CaMP2. Nature methods 12, 64–70 (2015). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
