This is a preprint.
Phase synchrony between prefrontal noradrenergic and cholinergic signals indexes inhibitory control
- PMID: 38798371
- PMCID: PMC11118516
- DOI: 10.1101/2024.05.17.594562
Phase synchrony between prefrontal noradrenergic and cholinergic signals indexes inhibitory control
Update in
-
Phase synchrony between prefrontal noradrenergic and cholinergic signals indexes inhibitory control.Nat Commun. 2025 Aug 6;16(1):7260. doi: 10.1038/s41467-025-62317-5. Nat Commun. 2025. PMID: 40770025 Free PMC article.
Abstract
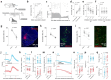
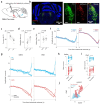
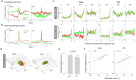
Inhibitory control is a critical executive function that allows animals to suppress their impulsive behavior in order to achieve certain goals or avoid punishment. We investigated norepinephrine (NE) and acetylcholine (ACh) dynamics and population neuronal activity in the prefrontal cortex (PFC) during inhibitory control. Using fluorescent sensors to measure extracellular levels of NE and ACh, we simultaneously recorded prefrontal NE and ACh dynamics in mice performing inhibitory control tasks. The prefrontal NE and ACh signals exhibited strong coherence at 0.4-0.8 Hz. Although inhibition of locus coeruleus (LC) neurons projecting to the PFC impaired inhibitory control, inhibiting LC neurons projecting to the basal forebrain (BF) caused a more profound impairment, despite an approximately 30% overlap between LC neurons projecting to the PFC and BF, as revealed by our tracing studies. The inhibition of LC neurons projecting to the BF did not diminish the difference in prefrontal NE/ACh signals between successful and failed trials; instead, it abolished the difference in NE-ACh phase synchrony between successful and failed trials, indicating that NE-ACh phase synchrony is a task-relevant neuromodulatory feature. Chemogenetic inhibition of cholinergic neurons that project to the LC region did not impair inhibitory control, nor did it abolish the difference in NE-ACh phase synchrony between successful or failed trials, further confirming the relevance of NE-ACh phase synchrony to inhibitory control. To understand the possible effect of NE-ACh synchrony on prefrontal population activity, we employed Neuropixels to record from the PFC during inhibitory control. The inhibition of LC neurons projecting to the BF not only reduced the number of prefrontal neurons encoding inhibitory control, but also disrupted population firing patterns representing inhibitory control, as revealed by a demixed principal component (dPCA) analysis. Taken together, these findings suggest that the LC modulates inhibitory control through its collective effect with cholinergic systems on population activity in the prefrontal cortex. Our results further indicate that NE-ACh phase synchrony is a critical neuromodulatory feature with important implications for cognitive control.
Keywords: Locus coeruleus; basal forebrain; cholinergic system; impulsivity; inhibitory control; noradrenergic system; prefrontal cortex; pupil.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
