This is a preprint.
The mutagenic forces shaping the genomic landscape of lung cancer in never smokers
- PMID: 38798417
- PMCID: PMC11118654
- DOI: 10.1101/2024.05.15.24307318
The mutagenic forces shaping the genomic landscape of lung cancer in never smokers
Abstract
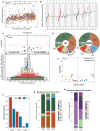
Lung cancer in never smokers (LCINS) accounts for up to 25% of all lung cancers and has been associated with exposure to secondhand tobacco smoke and air pollution in observational studies. Here, we evaluate the mutagenic exposures in LCINS by examining deep whole-genome sequencing data from a large international cohort of 871 treatment-naïve LCINS recruited from 28 geographical locations within the Sherlock-Lung study. KRAS mutations were 3.8-fold more common in adenocarcinomas of never smokers from North America and Europe, while a 1.6-fold higher prevalence of EGFR and TP53 mutations was observed in adenocarcinomas from East Asia. Signature SBS40a, with unknown cause, was found in most samples and accounted for the largest proportion of single base substitutions in adenocarcinomas, being enriched in EGFR-mutated cases. Conversely, the aristolochic acid signature SBS22a was almost exclusively observed in patients from Taipei. Even though LCINS exposed to secondhand smoke had an 8.3% higher mutational burden and 5.4% shorter telomeres, passive smoking was not associated with driver mutations in cancer driver genes or the activities of individual mutational signatures. In contrast, patients from regions with high levels of air pollution were more likely to have TP53 mutations while exhibiting shorter telomeres and an increase in most types of somatic mutations, including a 3.9-fold elevation of signature SBS4 (q-value=3.1 × 10-5), previously linked mainly to tobacco smoking, and a 76% increase of clock-like signature SBS5 (q-value=5.0 × 10-5). A positive dose-response effect was observed with air pollution levels, which correlated with both a decrease in telomere length and an elevation in somatic mutations, notably attributed to signatures SBS4 and SBS5. Our results elucidate the diversity of mutational processes shaping the genomic landscape of lung cancer in never smokers.
Conflict of interest statement
COMPETING INTERESTS LBA is a co-founder, CSO, scientific advisory member, and consultant for io9, has equity and receives income. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. LBA is also a compensated member of the scientific advisory board of Inocras. LBA’s spouse is an employee of Biotheranostics. ENB and LBA declare U.S. provisional patent application filed with UCSD with serial numbers 63/269,033. LBA also declares U.S. provisional applications filed with UCSD with serial numbers: 63/366,392; 63/289,601; 63/483,237; 63/412,835; and 63/492,348. LBA is also an inventor of a US Patent 10,776,718 for source identification by non-negative matrix factorization. SRY has received consulting fees from AstraZeneca, Sanofi, Amgen, AbbVie, and Sanofi; received speaking fees from AstraZeneca, Medscape, PRIME Education, and Medical Learning Institute. All other authors declare that they have no competing interests.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
