This is a preprint.
Sleep-wake body temperature regulates tau secretion in mice and correlates with CSF and plasma tau in humans
- PMID: 38798432
- PMCID: PMC11118695
- DOI: 10.21203/rs.3.rs-4384494/v1
Sleep-wake body temperature regulates tau secretion in mice and correlates with CSF and plasma tau in humans
Update in
-
Sleep-wake variation in body temperature regulates tau secretion and correlates with CSF and plasma tau.J Clin Invest. 2025 Feb 4;135(7):e182931. doi: 10.1172/JCI182931. J Clin Invest. 2025. PMID: 39903530 Free PMC article.
Abstract
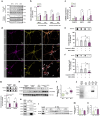
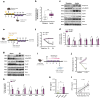
The sleep-wake cycle regulates interstitial fluid and cerebrospinal fluid (CSF) tau levels in both mouse and human by mechanisms that remain unestablished. Here, we reveal a novel pathway by which wakefulness increases extracellular tau levels in mouse and humans. In mice, higher body temperature (BT) associated with wakefulness and sleep deprivation increased CSF tau. In vitro, wakefulness temperatures upregulated tau secretion via a temperature-dependent increase in activity and expression of unconventional protein secretion pathway-1 components, namely caspase-3-mediated C-terminal cleavage of tau (TauC3), and membrane expression of PIP2 and syndecan-3. In humans, the increase in both CSF and plasma tau levels observed post-wakefulness correlated with BT increase during wakefulness. Our findings suggest sleep-wake variation in BT may contribute to regulating extracellular tau levels, highlighting the importance of thermoregulation in pathways linking sleep disturbance to neurodegeneration, and the potential for thermal intervention to prevent or delay tau-mediated neurodegeneration.
Keywords: Alzheimer’s disease; body temperature; sleep-wake cycle; tau; unconventional protein secretion.
Conflict of interest statement
Competing interests: All authors declare no competing interests.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

