This is a preprint.
Exportin-1 functions as an adaptor for transcription factor-mediated docking of chromatin at the nuclear pore complex
- PMID: 38798450
- PMCID: PMC11118273
- DOI: 10.1101/2024.05.09.593355
Exportin-1 functions as an adaptor for transcription factor-mediated docking of chromatin at the nuclear pore complex
Update in
-
Exportin-1 functions as an adaptor for transcription factor-mediated docking of chromatin at the nuclear pore complex.Mol Cell. 2025 Mar 20;85(6):1101-1116.e8. doi: 10.1016/j.molcel.2025.02.013. Epub 2025 Mar 10. Mol Cell. 2025. PMID: 40068679
Abstract
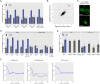
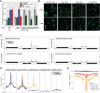
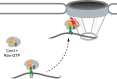
Nuclear pore proteins (Nups) in yeast, flies and mammals physically interact with hundreds or thousands of chromosomal sites, which impacts transcriptional regulation. In budding yeast, transcription factors mediate interaction of Nups with enhancers of highly active genes. To define the molecular basis of this mechanism, we exploited a separation-of-function mutation in the Gcn4 transcription factor that blocks its interaction with the nuclear pore complex (NPC) without altering its DNA binding or activation domains. SILAC mass spectrometry revealed that this mutation reduces the interaction of Gcn4 with the highly conserved nuclear export factor Crm1/Xpo1. Crm1 both interacts with the same sites as Nups genome-wide and is required for Nup2 to interact with the yeast genome. In vivo, Crm1 undergoes extensive and stable interactions with the NPC. In vitro, Crm1 binds to Gcn4 and these proteins form a complex with the nuclear pore protein Nup2. Importantly, the interaction between Crm1 and Gcn4 does not require Ran-GTP, suggesting that it is not through the nuclear export sequence binding site. Finally, Crm1 stimulates DNA binding by Gcn4, supporting a model in which allosteric coupling between Crm1 binding and DNA binding permits docking of transcription factor-bound enhancers at the NPC.
Figures




Similar articles
-
Exportin-1 functions as an adaptor for transcription factor-mediated docking of chromatin at the nuclear pore complex.Mol Cell. 2025 Mar 20;85(6):1101-1116.e8. doi: 10.1016/j.molcel.2025.02.013. Epub 2025 Mar 10. Mol Cell. 2025. PMID: 40068679
-
A role for RanBP1 in the release of CRM1 from the nuclear pore complex in a terminal step of nuclear export.J Cell Biol. 1999 May 17;145(4):645-57. doi: 10.1083/jcb.145.4.645. J Cell Biol. 1999. PMID: 10330396 Free PMC article.
-
NXT1 is necessary for the terminal step of Crm1-mediated nuclear export.J Cell Biol. 2001 Jan 8;152(1):141-55. doi: 10.1083/jcb.152.1.141. J Cell Biol. 2001. PMID: 11149927 Free PMC article.
-
Mechanistic insights from the recent structures of the CRM1 nuclear export complex and its disassembly intermediate.Biophysics (Nagoya-shi). 2012 Nov 30;8:145-50. doi: 10.2142/biophysics.8.145. eCollection 2012. Biophysics (Nagoya-shi). 2012. PMID: 27493531 Free PMC article. Review.
-
The Nuclear Pore Complex as a Transcription Regulator.Cold Spring Harb Perspect Biol. 2022 Jan 4;14(1):a039438. doi: 10.1101/cshperspect.a039438. Cold Spring Harb Perspect Biol. 2022. PMID: 34127448 Free PMC article. Review.
References
-
- Gozalo A., Duke A., Lan Y., Pascual-Garcia P., Talamas J.A., Nguyen S.C., Shah P.P., Jain R., Joyce E.F., and Capelson M. (2020). Core Components of the Nuclear Pore Bind Distinct States of Chromatin and Contribute to Polycomb Repression. Mol Cell 77, 67–81.e7. 10.1016/j.molcel.2019.10.017. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
