This is a preprint.
Inhibition of mTORC1 by rapamycin results in feedback activation of AktS473 and aggravates hallmarks of osteoarthritis in female mice and non-human primates
- PMID: 38798488
- PMCID: PMC11118493
- DOI: 10.1101/2024.05.14.594256
Inhibition of mTORC1 by rapamycin results in feedback activation of AktS473 and aggravates hallmarks of osteoarthritis in female mice and non-human primates
Abstract
Purpose: Genetic deletion of mTOR has protected against post-traumatic osteoarthritis (OA) in male mice, however, effects of pharmacological mTOR-inhibition are equivocal and have not been tested in aging models nor in female subjects. Therefore, the goal of this study was to determine if mTOR-inhibition by rapamycin can modify OA pathology in aging non-human primates and female mice.
Methods: Common marmosets were administered oral rapamycin (1mg/kg/day) or vehicle starting near mid-life until death. Five-month-old, female C57BL/6J mice were treated with vehicle or rapamycin (IP, 2mg/kg, 3x/week) for 8-weeks following non-invasive ACL rupture. Knee OA pathology was assessed via microCT and histology. Phosphorylation of mTORC1 (p-RPS6S235/36) and mTORC2 (p-AktS473, p-NDRG1T638, p-PKCαT348) substrates were evaluated via western blot in articular cartilage, meniscus, and/or infrapatellar fat pad. ATDC5 cells were cultured with rapamycin to determine time and dose effects on mTORC1/2 signaling.
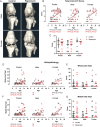
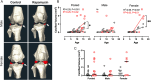
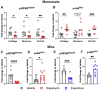
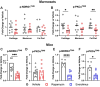
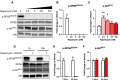
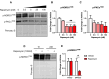
Results: In marmosets, rapamycin did not impact age-related radiographic OA severity or cartilage pathology but increased medial meniscus calcification and lowered lateral tibia subchondral thickness, particularly in females. In female mice, rapamycin worsened ACLR-induced meniscus calcification and cartilage pathology. In marmoset and mouse joint tissues, rapamycin inhibited mTORC1 and increased p-AktS473 but not p-NDRG1T638 or p-PKCαT348. This mTOR signaling pattern was replicated in ATDC5 cells during exposure to low concentrations of rapamycin.
Conclusions: Rapamycin attenuated mTORC1 signaling with feedback activation of AktS473 in articular cartilage, meniscus, and/or infrapatellar fat pad and was accompanied by deleterious effects on meniscus calcification and/or cartilage pathology in female mice and common marmosets.
Conflict of interest statement
Conflicts of Interests DWL has received funding from, and is a scientific advisory board member of, Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases.
Figures


Similar articles
-
Reduced menin expression impairs rapamycin effects as evidenced by an increase in mTORC2 signaling and cell migration.Cell Commun Signal. 2018 Oct 1;16(1):64. doi: 10.1186/s12964-018-0278-2. Cell Commun Signal. 2018. PMID: 30285764 Free PMC article.
-
The common marmoset as a translational model of age-related osteoarthritis.Geroscience. 2024 Jun;46(3):2827-2847. doi: 10.1007/s11357-024-01103-5. Epub 2024 Mar 11. Geroscience. 2024. PMID: 38466454 Free PMC article.
-
Increased AKT S473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition.Mol Cancer Ther. 2009 Apr;8(4):742-53. doi: 10.1158/1535-7163.MCT-08-0668. Mol Cancer Ther. 2009. PMID: 19372546 Free PMC article.
-
Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2.PLoS Biol. 2009 Feb 10;7(2):e38. doi: 10.1371/journal.pbio.1000038. PLoS Biol. 2009. PMID: 19209957 Free PMC article.
-
Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship.Adv Biol Regul. 2019 May;72:51-62. doi: 10.1016/j.jbior.2019.03.003. Epub 2019 Apr 11. Adv Biol Regul. 2019. PMID: 31010692 Review.
References
Publication types
Grants and funding
- R56 AG056771/AG/NIA NIH HHS/United States
- R21 AG067464/AG/NIA NIH HHS/United States
- R01 AG056771/AG/NIA NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- S10 OD023526/OD/NIH HHS/United States
- R01 AG050797/AG/NIA NIH HHS/United States
- T32 AG000213/AG/NIA NIH HHS/United States
- RF1 AG056771/AG/NIA NIH HHS/United States
- P30 AG044271/AG/NIA NIH HHS/United States
- R01 DK125859/DK/NIDDK NIH HHS/United States
- P51 OD011133/OD/NIH HHS/United States
- U01 AG076941/AG/NIA NIH HHS/United States
- R01 AG085898/AG/NIA NIH HHS/United States
- U01 AG081482/AG/NIA NIH HHS/United States
- R01 AG084156/AG/NIA NIH HHS/United States
- P30 AG013319/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous
