This is a preprint.
Gut commensals require Peyer's patches to induce protective systemic IgA responses
- PMID: 38798510
- PMCID: PMC11118714
- DOI: 10.21203/rs.3.rs-4220532/v1
Gut commensals require Peyer's patches to induce protective systemic IgA responses
Abstract
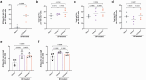
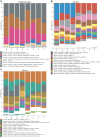
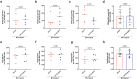
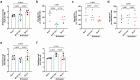
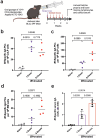
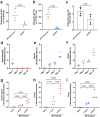
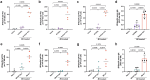
Gut educated IgA secreting plasma cells that disseminate beyond the mucosa and into systemic tissues have been described as providing beneficial effects from disease in several contexts. Several bacteria have been implicated in the induction of systemic IgA, however the mechanisms that result in differential levels of induction by each bacterial species are still unknown. Here we show, the commensal bacteria, Bacteroides fragilis (Bf), is an efficient inducer of systemic IgA responses. The ability of Bf to induce the production of bone marrow IgA plasma cells and high levels of serum IgA relied on high levels of gut colonization in a dose-dependent manner. Colonization induced Bf-specific IgA responses were severely diminished in the absence of Peyer's patches, but not the murine cecal patch. Colonization of mice with Bf, a natural human commensal, resulted in few changes within the microbiome and the host transcriptional profile in the gut, suggesting a commensal relationship with the host. Bf colonization did benefit the mice by inducing systemic IgA that led to increased protection in a bowel perforation model resulting in lower peritoneal abscess formation. These findings demonstrate a critical role for bacterial colonization and Peyer's patches in the induction of robust systemic IgA responses that confer protection from bacterial dissemination outside of the gut.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures







Similar articles
-
Bacterial and host fucosylation maintain IgA homeostasis to limit intestinal inflammation in mice.Nat Microbiol. 2025 Jan;10(1):126-143. doi: 10.1038/s41564-024-01873-w. Epub 2024 Dec 17. Nat Microbiol. 2025. PMID: 39690194
-
Bacteroides induce higher IgA production than Lactobacillus by increasing activation-induced cytidine deaminase expression in B cells in murine Peyer's patches.Biosci Biotechnol Biochem. 2009 Feb;73(2):372-7. doi: 10.1271/bbb.80612. Epub 2009 Feb 7. Biosci Biotechnol Biochem. 2009. PMID: 19202287
-
Mouse and Human Liver Contain Immunoglobulin A-Secreting Cells Originating From Peyer's Patches and Directed Against Intestinal Antigens.Gastroenterology. 2016 Aug;151(2):311-23. doi: 10.1053/j.gastro.2016.04.014. Epub 2016 Apr 27. Gastroenterology. 2016. PMID: 27132185
-
The secretory IgA system of the gut.Ciba Found Symp. 1977 Apr 26-28;(46):5-28. doi: 10.1002/9780470720288.ch2. Ciba Found Symp. 1977. PMID: 346328 Review.
-
Mucosal immunoregulation: environmental lipopolysaccharide and GALT T lymphocytes regulate the IgA response.Microbiol Immunol. 1984;28(3):261-80. doi: 10.1111/j.1348-0421.1984.tb00679.x. Microbiol Immunol. 1984. PMID: 6234450 Review.
References
-
- Usami K. et al. The gut microbiota induces Peyer’s-patch-dependent secretion of maternal IgA into milk. Cell Reports 36, 109655 (2021). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

