This is a preprint.
Multi-organ structural homogeneity of amyloid fibrils in ATTRv-T60A amyloidosis patients, revealed by Cryo-EM
- PMID: 38798519
- PMCID: PMC11118364
- DOI: 10.1101/2024.05.14.594218
Multi-organ structural homogeneity of amyloid fibrils in ATTRv-T60A amyloidosis patients, revealed by Cryo-EM
Abstract
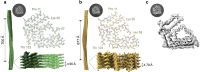
ATTR amyloidosis is a degenerative disorder characterized by the systemic deposition of the protein transthyretin. These amyloid aggregates of transthyretin (ATTR) can deposit in different parts of the body causing diverse clinical manifestations. Our laboratory aims to investigate a potential relationship between the different genotypes, organ of deposition, clinical phenotypes, and the structure of ATTR fibrils. Using cryo-electron microscopy, we have recently described how the neuropathic related mutations ATTRv-I84S and ATTRv-V122∆ can drive structural polymorphism in ex vivo fibrils. Here we question whether the mutation ATTRv-T60A, that commonly triggers cardiac and neuropathic symptoms, has a similar effect. To address this question, we extracted and determined the structure of ATTR-T60A fibrils from multiple organs (heart, thyroid, kidney, and liver) from the same patient and from the heart of two additional patients. We have found a consistent conformation among all the fibril structures, acquiring the "closed-gate morphology" previously found in ATTRwt and others ATTRv related to cardiac or mixed manifestations. The closed-gate morphology is composed by two segments of the protein that interact together forming a polar channel, where the residues glycine 57 to isoleucine 68 act as a gate of the polar cavity. Our study indicates that ATTR-T60A fibrils present in peripheral organs adopt the same structural conformation in all patients, regardless of the organ of deposition.
Figures

Similar articles
-
Cryo-EM confirms a common fibril fold in the heart of four patients with ATTRwt amyloidosis.bioRxiv [Preprint]. 2024 Mar 9:2024.03.08.582936. doi: 10.1101/2024.03.08.582936. bioRxiv. 2024. Update in: Commun Biol. 2024 Jul 27;7(1):905. doi: 10.1038/s42003-024-06588-6. PMID: 38496656 Free PMC article. Updated. Preprint.
-
Structural polymorphism of amyloid fibrils in ATTR amyloidosis revealed by cryo-electron microscopy.Nat Commun. 2024 Jan 17;15(1):581. doi: 10.1038/s41467-024-44820-3. Nat Commun. 2024. PMID: 38233397 Free PMC article.
-
A Shared Amyloid Architecture in Cardiac Fibrils from Three Neuropathy-Associated ATTR Variants.bioRxiv [Preprint]. 2025 Aug 7:2025.08.05.667944. doi: 10.1101/2025.08.05.667944. bioRxiv. 2025. PMID: 40799554 Free PMC article. Preprint.
-
Amyloid myopathy: expanding the clinical spectrum of transthyretin amyloidosis-case report and literature review.J Nucl Cardiol. 2023 Aug;30(4):1420-1426. doi: 10.1007/s12350-022-02990-x. Epub 2022 May 17. J Nucl Cardiol. 2023. PMID: 35581484 Free PMC article. Review.
-
Ultrastructure in Transthyretin Amyloidosis: From Pathophysiology to Therapeutic Insights.Biomedicines. 2019 Feb 5;7(1):11. doi: 10.3390/biomedicines7010011. Biomedicines. 2019. PMID: 30764529 Free PMC article. Review.
References
-
- Adams D., Ando Y., Beirão J. M., Coelho T., Gertz M. A., Gillmore J. D., Hawkins P. N., Lousada I., Suhr O. B., & Merlini G. (2021). Expert consensus recommendations to improve diagnosis of ATTR amyloidosis with polyneuropathy. J Neurol, 268(6), 2109–2122. 10.1007/s00415-019-09688-0 - DOI - PMC - PubMed
-
- Allinovi M., Bergesio F., Cappelli F., Chiappini M. G., Santostefano M., Argirò A., Catalucci T., Parise A., Zampieri M., & Perfetto F. (2022). Is Hereditary Transthyretin Amyloidosis the Third Leading Cause of Monogenic Chronic Kidney Disease, Only Behind ADPKD and Alport Disease? Am J Nephrol, 53(8–9), 624–627. 10.1159/000526955 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
