This is a preprint.
Structural Basis of Aquaporin-4 Autoantibody Binding in Neuromyelitis Optica
- PMID: 38798537
- PMCID: PMC11118524
- DOI: 10.1101/2024.05.12.592631
Structural Basis of Aquaporin-4 Autoantibody Binding in Neuromyelitis Optica
Update in
-
Structural basis of aquaporin-4 autoantibody binding in neuromyelitis optica.Sci Adv. 2025 Feb 21;11(8):eadq7560. doi: 10.1126/sciadv.adq7560. Epub 2025 Feb 21. Sci Adv. 2025. PMID: 39982991 Free PMC article.
Abstract
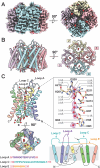
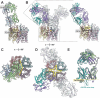
Neuromyelitis Optica (NMO) is an autoimmune disease of the central nervous system where pathogenic autoantibodies target the human astrocyte water channel aquaporin-4 causing neurological impairment. Autoantibody binding leads to complement dependent and complement independent cytotoxicity, ultimately resulting in astrocyte death, demyelination, and neuronal loss. Aquaporin-4 assembles in astrocyte plasma membranes as symmetric tetramers or as arrays of tetramers. We report molecular structures of aquaporin-4 alone and bound to Fab fragments from patient-derived NMO autoantibodies using cryogenic electron microscopy. Each antibody binds to epitopes comprised of three extracellular loops of aquaporin-4 with contributions from multiple molecules in the assembly. The structures distinguish between antibodies that bind to the tetrameric form of aquaporin-4, and those targeting higher order orthogonal arrays of tetramers that provide more diverse bridging epitopes.
Conflict of interest statement
Competing interests: Authors declare that they have no competing interests.
Figures



References
-
- Jarius S. et al., Neuromyelitis optica. Nat Rev Dis Primers 6, 85 (2020). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
