Progesterone Enhances Niraparib Efficacy in Ovarian Cancer by Promoting Palmitoleic-Acid-Mediated Ferroptosis
- PMID: 38798714
- PMCID: PMC11116976
- DOI: 10.34133/research.0371
Progesterone Enhances Niraparib Efficacy in Ovarian Cancer by Promoting Palmitoleic-Acid-Mediated Ferroptosis
Abstract
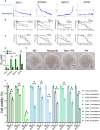
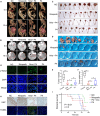
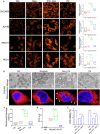
Poly (adenosine 5'-diphosphate-ribose) polymerase inhibitors (PARPi) are increasingly important in the treatment of ovarian cancer. However, more than 40% of BRCA1/2-deficient patients do not respond to PARPi, and BRCA wild-type cases do not show obvious benefit. In this study, we demonstrated that progesterone acted synergistically with niraparib in ovarian cancer cells by enhancing niraparib-mediated DNA damage and death regardless of BRCA status. This synergy was validated in an ovarian cancer organoid model and in vivo experiments. Furthermore, we found that progesterone enhances the activity of niraparib in ovarian cancer through inducing ferroptosis by up-regulating palmitoleic acid and causing mitochondrial damage. In clinical cohort, it was observed that progesterone prolonged the survival of patients with ovarian cancer receiving PARPi as second-line maintenance therapy, and high progesterone receptor expression combined with low glutathione peroxidase 4 (GPX4) expression predicted better efficacy of PARPi in patients with ovarian cancer. These findings not only offer new therapeutic strategies for PARPi poor response ovarian cancer but also provide potential molecular markers for predicting the PARPi efficacy.
Copyright © 2024 Nayiyuan Wu et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

