Atezolizumab/bevacizumab or lenvatinib in hepatocellular carcinoma: Multicenter real-world study with focus on bleeding and thromboembolic events
- PMID: 38798717
- PMCID: PMC11126929
- DOI: 10.1016/j.jhepr.2024.101065
Atezolizumab/bevacizumab or lenvatinib in hepatocellular carcinoma: Multicenter real-world study with focus on bleeding and thromboembolic events
Abstract
Background & aims: Atezolizumab/bevacizumab (atezo/bev) and lenvatinib have demonstrated efficacy as first-line therapies for hepatocellular carcinoma (HCC). However, vascular endothelial growth factor (VEGF) inhibition with these therapies may be associated with the risk of bleeding and thromboembolic events. In this study, we evaluated the efficacy and safety with focus on the bleeding and thromboembolic events of atezo/bev vs. lenvatinib in a large, multicenter real-world population.
Methods: This study is based on HCC cohorts from seven centers in Germany and Austria. Incidences of bleeding or thromboembolic events and efficacy outcomes were assessed and compared.
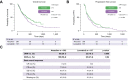
Results: In total, 464 patients treated with atezo/bev (n = 325) or lenvatinib (n = 139) were analyzed. Both groups were balanced with respect to demographics, presence of liver cirrhosis, and variceal status. Duration of therapy did not differ between groups. Within 3 months of therapy, bleeding episodes were described in 57 (18%) patients receiving atezo/bev compared with 15 (11%) patients receiving lenvatinib (p = 0.07). Variceal hemorrhage occurred in 11 (3%) patients treated with atezo/bev compared with 4 (3%) patients treated with lenvatinib (p = 0.99). Thromboembolic events were reported in 19 (6%) of patients in the atezo/bev cohort compared with 5 (4%) patients in the lenvatinib cohort (p = 0.37). In addition, incidence of overall bleeding, variceal hemorrhage, and thromboembolic events did not differ significantly in patients who received either atezo/bev or lenvantinib for 6 months.
Conclusions: Safety considerations related to bleeding and thromboembolic events may not be helpful in guiding clinical decision-making when choosing between atezo/bev and lenvatinib.
Impact and implications: The inhibition of VEGF by current first-line therapies for HCC, such as atezolizumab/bevacizumab or lenvatinib, may be associated with the risk of bleeding and thromboembolic events. Studies comparing the incidence of these side effects between atezolizumab/bevacizumab and lenvatinib, which are preferred treatments over sorafenib for HCC, are needed. Differences in this side effect profile may influence the choice of first-line therapy by treating physicians. Because no significant differences were observed regarding bleeding or thromboembolic events between both therapies in the present study, we conclude that safety considerations related to these events may not be helpful in guiding clinical decision-making when choosing between atezolizumab/bevacizumab and lenvatinib.
Keywords: Hepatocellular carcinoma; Immunotherapy; Tyrosine kinase inhibition.
© 2024 The Author(s).
Conflict of interest statement
NBK has received reimbursement of meeting attendance fees and travel expenses from EISAI and lecture honoraria from the Falk Foundation and AstraZeneca. UE has received honoraria for lectures from AstraZeneca, the Falk Foundation, Ipsen, and Novartis and travel support from AstraZeneca and Biotest. She has served as an advisory board or steering committee member to AstraZeneca, Bayer, EISAI, and MSD. KB has received honoraria for lectures from Ipsen. MP served as a speaker and/or consultant and/or advisory board member for AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, Ipsen, Lilly, MSD, and Roche and received travel support from Bayer, Bristol-Myers Squibb, Ipsen, and Roche. BS received grant support from AstraZeneca and Eisai; speaker honoraria from Eisai; and travel support from AbbVie, AstraZeneca, Ipsen and Gilead. OÖ received honorarium from Bayer. MV has received honoraria for her speaker, consultancy, and advisory roles from Amgen, AstraZeneca, Bayer, BMS, EISAI, Ipsen, Lilly, Merck Serono, MSD, Nordic Pharma, Roche, Servier, and Sirtex. SJG has received travel expenses from Gilead and Ipsen. FF has received honoraria for lectures from AstraZeneca, MSD, Pfizer, and Roche and reimbursement of meeting attendance fees and travel expenses from Merck KGaA and Servier. He has served as an advisory board or steering committee member to AstraZeneca, BMS, Eisai, and Roche. ENDT has served as a paid consultant for AstraZeneca, Bayer, BMS, EISAI, Eli Lilly & Co, Pfizer, Ipsen, and Roche. He has received reimbursement of meeting attendance fees and travel expenses from Arqule, AstraZeneca, BMS, Bayer, Celsion, and Roche and lecture honoraria from BMS and Falk Foundation. He has received third-party funding for scientific research from Arqule, AstraZeneca, BMS, Bayer, Eli Lilly, and Roche. AG is an advisory board or steering committee member to AbbVie, Alexion, Bayer, BMS, CSL Behring, Eisai, Falk, Gilead, Heel, Intercept, Ipsen, Merz, MSD, Novartis, Pfizer, Roche, Sanofi-Aventis, and Sequana and a speaker for Advanz. FPR has received honoraria for lectures, consulting activities, and travel support from the Falk Foundation, AbbVie, Gilead, Ipsen, AstraZeneca, Roche and Novartis. All other authors report no conflicts of interest. MM, LSJ, CL, LB, AW, HBL, VZ, LY, JS, IP, MR, FS, and JM have nothing to declare. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Bray F., Ferlay J., Soerjomataram I., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Llovet J.M., Kelley R.K., Villanueva A., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. - PubMed
LinkOut - more resources
Full Text Sources
Medical

